Argentine hemorrhagic fever
Topic: Medicine
 From HandWiki - Reading time: 9 min
From HandWiki - Reading time: 9 min
| Argentine hemorrhagic fever | |
|---|---|
 | |
| Specialty | Infectious disease |
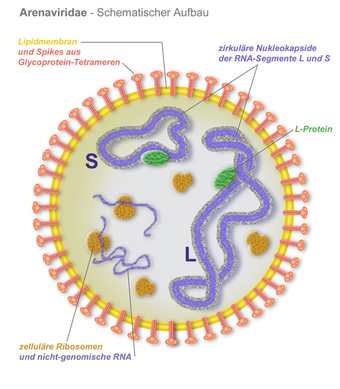
Argentine hemorrhagic fever (AHF) or O'Higgins disease, also known in Argentina as mal de los rastrojos (stubble disease) is a hemorrhagic fever and zoonotic infectious disease occurring in Argentina. It is caused by the Junín virus[1] (an arenavirus, closely related to the Machupo virus, causative agent of Bolivian hemorrhagic fever). Its reservoir of infection is the drylands vesper mouse, a rodent found in Argentina and Paraguay.
Epidemiology
The disease was first reported in the town of O'Higgins in Buenos Aires province, Argentina in 1958, giving it one of the names by which it is known.[2] Theories about its nature included: Weil's disease, leptospirosis, chemical pollution.[2] It was associated with fields containing stubble after the harvest, giving it another of its names.
The natural reservoir of infection, a small rodent known locally as ratón maicero ("maize mouse"; Calomys musculinus), has chronic asymptomatic infection, and spreads the virus through its saliva and urine. Infection is produced through contact of skin or mucous membranes, or through inhalation of infected particles. Human-to-human transmission is extremely uncommon but can occur through direct exposure to the bodily fluids of a viremic individual. Cases of hospital-acquired infections have also been documented.[3] Large outbreaks primarily take place during Argentina's harvesting season, with peak incidence in May. The disease is four times more common in males than females and occurs more frequently among rural workers than urban populations.[3]
Clinical aspects
AHF is a grave acute disease which may progress to recovery or death in 1 to 2 weeks. The incubation time of the disease is between 6 and 14 days, after which the first symptoms appear: fever, headaches, weakness, loss of appetite and will. These can rapidly progress to worsening gastrointestinal, cardiovascular and neurological symptoms.[4]
The illness progresses through three distinct phases: prodromal, neurologic-hemorrhagic, and convalescence.[4]
- Prodromal Phase: This initial stage lasts about a week and begins gradually with symptoms such as chills, fatigue, headache, muscle pain (especially in the lower back), and moderate fever. Additional symptoms may include eye pain, nausea, dizziness, and digestive disturbances. Patients often exhibit flushed skin, conjunctival congestion, and mild bleeding from the gums. Petechiae, lymph node swelling, and neurological signs like tremors and ataxia may develop.[4]
- Neurologic-Hemorrhagic Phase: Occurring in 20–30% of cases between days 8 and 12, this phase is marked by severe bleeding (e.g., gastrointestinal, respiratory, or urinary hemorrhages) and neurological deterioration, including confusion, ataxia, seizures, and coma. Secondary bacterial infections, such as pneumonia and sepsis, may complicate the illness. While acute kidney failure is rare, it can occur in severe cases due to prolonged shock.[4]
- Convalescence Phase: Recovery can take one to three months, with lingering fatigue, irritability, memory impairment, and hair loss. About 10% of those treated with immune plasma develop a delayed neurological syndrome characterized by fever, cerebellar dysfunction, and cranial nerve impairments. However, this syndrome has not been reported in untreated survivors.[4]
Reverse transcription–polymerase chain reaction (RT-PCR) is typically the most sensitive diagnostic method, generating amplicons that can be sequenced for genetic analysis.[5] If untreated, the mortality of AHF reaches 15–30%. The specific treatment includes plasma of recovered patients, which, if started early, is extremely effective and reduces mortality to 1%.[6]
Ribavirin also has shown some promise in treating arenaviral diseases.
The disease was first detected in the 1950s in the Junín Partido in Buenos Aires, after which its agent, the Junín virus, was named upon its identification in 1958. In the early years, about 1,000 cases per year were recorded, with a high mortality rate (more than 30%). The initial introduction of treatment serums in the 1970s reduced this lethality.
Vaccine Development and Public Health Impact
The Candid #1 vaccine for AHF was created in 1985 by Argentine virologist Dr. Julio Barrera Oro. The vaccine was manufactured by the Salk Institute in the United States, and became available in Argentina in 1990. Antibodies produced by Candid #1 vaccination have also demonstrated cross-reactivity with Machupo virus in Rhesus macaques, and thus Candid #1 been considered for prophylactic use against Bolivian hemorrhagic fever.[7]
Clinical trials have demonstrated that Candid #1 is both safe and highly efficacious, with no serious adverse events attributed to the vaccination. It has been applied to adult high-risk population and is 95.5% effective.[8] Between 1991 and 2005 more than 240,000 people were vaccinated, achieving a great decrease in the numbers of reported cases (94 suspect and 19 confirmed in 2005). Prior to widespread vaccination, AHF posed a substantial public health threat, with high case-fatality rates and considerable economic impact due to its effect on the agricultural workforce.[9]The implementation of vaccination programs has led to a marked decrease in cases, underscoring the vaccine's effectiveness.
In addition to vaccination, Argentina established a National Program for the control of AHF, emphasizing the early use of immune plasma from recovered patients as a standard treatment. This program included the development of plasma banks with certified quality, further reducing the case-fatality rate associated with AHF.
On 29 August 2006 the Maiztegui Institute obtained certification for the production of the vaccine in Argentina. The vaccine produced in Argentina was found to be of similar effectiveness to the US vaccine.[10] Details of the vaccine were published in 2011,[8] and a protocol for production of the vaccine was published in 2018.[11] Demand for the vaccine is insufficient to be commercially appealing due to the small target population, and it is considered an orphan drug; the Argentine government committed itself to manufacture and sponsor Candid #1 vaccine.[8] Since 2003, Argentina's Instituto Nacional de Enfermedades Virales Humanas (INEVH) has been producing the Candid #1 vaccine, ensuring its stability and effectiveness under various conditions.[12]
One Health Approach
The appearance of AHF in the 1950s is believed to be linked to human modifications of the environment due to agricultural activities. These changes are thought to have facilitated the population expansion of C. musculinus.[13]
A One Health approach is critical to understanding and controlling AHF. The interplay of environmental, agricultural, and human behavioral factors influences viral transmission. The ongoing global changes, such as land-use modifications and urbanization, create favorable conditions for various rodent species to expand beyond their natural habitats, largely due to their close association with human environments.[14] Integrated surveillance of rodents, ecological assessments, and public health initiatives are necessary to mitigate outbreaks.
To effectively manage these diseases, the One Health approach focuses on two key strategies:
- Rodent Surveillance: Comprehensive monitoring of rodent populations, especially Calomys musculinus, which serves as the primary reservoir for Junín virus, is crucial in understanding virus transmission patterns. Enhanced surveillance can inform targeted public health interventions.[15]
- Rodent Control Measures: Implementing effective rodent control strategies, such as trapping, safe food storage, and habitat modification, can significantly reduce human exposure to infected rodents, decreasing the risk of outbreaks.[15]
Implementing a holistic One Health strategy for SAHF may enhance efforts to prevent spillover events and manage outbreaks more effectively.[15] Additionally, continued public health efforts, including surveillance, vaccination, and treatment programs, remain crucial in managing AHF and mitigating its impact on affected communities.
Weaponization
Argentine hemorrhagic fever was one of three hemorrhagic fevers and one of more than a dozen agents that the United States researched as potential biological weapons before the nation suspended its biological weapons program.[16] The Soviet Union also conducted research and developing programs on the potential of the hemorragic fever as a biological weapon.[17]
References
- ↑ "Junín virus pathogenesis and virus replication". Viruses (National Institutes of Health) 4 (10): 2317–2339. October 2012. doi:10.3390/v4102317. PMID 23202466. "Junín virus, the etiological agent of Argentine hemorrhagic fever, causes significant morbidity and mortality.".
- ↑ 2.0 2.1 "Una rara enfermedad alarma a la modesta población de O'Higgins. Análisis del discurso de la prensa escrita sobre la epidemia de Fiebre Hemorrágica Argentina de 1958" (in Spanish). Revista de Historia & Humanidades Médicas 3 (1). July 2007. http://www.fmv-uba.org.ar/histomedicina_old/Articulo%20-%20Fiebre%20Hemorragica%20Argentina%20de%201958.pdf.
- ↑ 3.0 3.1 Grant, Ashley; Seregin, Alexey; Huang, Cheng; Kolokoltsova, Olga; Brasier, Allan; Peters, Clarence; Paessler, Slobodan (2012-10-19). "Junín Virus Pathogenesis and Virus Replication" (in en). Viruses 4 (10): 2317–2339. doi:10.3390/v4102317. ISSN 1999-4915. PMID 23202466.
- ↑ 4.0 4.1 4.2 4.3 4.4 Enria, Delia A.; Briggiler, Ana M.; Sánchez, Zaida (April 2008). "Treatment of Argentine hemorrhagic fever" (in en). Antiviral Research 78 (1): 132–139. doi:10.1016/j.antiviral.2007.10.010. PMID 18054395.
- ↑ Grant, Ashley; Seregin, Alexey; Huang, Cheng; Kolokoltsova, Olga; Brasier, Allan; Peters, Clarence; Paessler, Slobodan (2012-10-19). "Junín Virus Pathogenesis and Virus Replication" (in en). Viruses 4 (10): 2317–2339. doi:10.3390/v4102317. ISSN 1999-4915. PMID 23202466.
- ↑ "The Use of Ebola Convalescent Plasma to Treat Ebola Virus Disease in Resource-Constrained Settings: A Perspective From the Field". Clinical Infectious Diseases 62 (1): 69–74. January 2016. doi:10.1093/cid/civ680. PMID 26261205.
- ↑ "Comparative analysis of disease pathogenesis and molecular mechanisms of New World and Old World arenavirus infections". The Journal of General Virology 95 (Pt 1): 1–15. January 2014. doi:10.1099/vir.0.057000-0. PMID 24068704.
- ↑ 8.0 8.1 8.2 "Argentine hemorrhagic fever vaccines". Human Vaccines 7 (6): 694–700. June 2011. doi:10.4161/hv.7.6.15198. PMID 21451263.
- ↑ Pan American Health Organization (PAHO), Thousand Oaks, California: SAGE Publications, Inc., 2008, doi:10.4135/9781412963855.n931, ISBN 978-1-4129-4186-0, https://doi.org/10.4135/9781412963855.n931, retrieved 2025-03-19
- ↑ "Vacuna contra la fiebre hemorrágica argentina Candid#1 producida en la Argentina. Inmunogenicidad y seguridad". MEDICINA (Buenos Aires) 70: 215–222. 2010. https://www.rosario.gob.ar/mr/epidemiologia/vigilancia/vigilancia-intensificada/fiebre-hemorragica-argentina-f-h-a/vacuna-contra-la-fiebre-hemorragica-argentina-candid-1-producida-en-la-argentina-inmunogenicidad-y-seguridad/view. Article in Spanish with abstract in English.
- ↑ "Protocol for the Production of a Vaccine Against Argentinian Hemorrhagic Fever". Hemorrhagic Fever Viruses. Methods in Molecular Biology. 1604. 2018. pp. 305–329. doi:10.1007/978-1-4939-6981-4_24. ISBN 978-1-4939-6980-7.
- ↑ Grant, Ashley; Seregin, Alexey; Huang, Cheng; Kolokoltsova, Olga; Brasier, Allan; Peters, Clarence; Paessler, Slobodan (2012-10-19). "Junín Virus Pathogenesis and Virus Replication" (in en). Viruses 4 (10): 2317–2339. doi:10.3390/v4102317. ISSN 1999-4915. PMID 23202466.
- ↑ "A Narrative Review on Argentine Hemorrhagic Fever: Junin Virus (JUNV)". J Clin Immunol Microbiol 4 (2): 1–4. 17 July 2023. https://athenaeumpub.com/a-narrative-review-on-argentine-hemorrhagic-fever-junin-virus-junv-2/.
- ↑ Dahmana, Handi; Granjon, Laurent; Diagne, Christophe; Davoust, Bernard; Fenollar, Florence; Mediannikov, Oleg (2020-03-10). "Rodents as Hosts of Pathogens and Related Zoonotic Disease Risk" (in en). Pathogens 9 (3): 202. doi:10.3390/pathogens9030202. ISSN 2076-0817. PMID 32164206.
- ↑ 15.0 15.1 15.2 Wilson, Dr Rory; Barry, Dr Ariella (2025-03-03). "A One Health approach for South American hemorrhagic fevers" (in en). CABI One Health. doi:10.1079/cabionehealth.2025.0008. ISSN 2791-223X. http://www.cabidigitallibrary.org/doi/10.1079/cabionehealth.2025.0008.
- ↑ ""Chemical and Biological Weapons: Possession and Programs Past and Present". James Martin Center for Nonproliferation Studies. Middlebury College. 9 April 2002. http://www.cns.miis.edu/research/cbw/possess.htm.
- ↑ Deadly cultures: biological weapons since 1945. Cambridge, Mass.: Harvard University Press. 2006. pp. 141. ISBN 0-674-01699-8.
Further reading
- "Argentina fabricará vacuna contra la fiebre hemorrágica" (in Spanish). Argentine Ministry of Health and Environment. 8 October 2006. http://www.msal.gov.ar/htm/site/Noticias_plantilla.asp?Id=915.
- "La vacuna contra el mal de los rastrojos ya se puede elaborar en el país" (in Spanish). Clarín. 29 September 2006. http://www.clarin.com/diario/2006/09/29/sociedad/s-03801.htm.
- "Fiebre Hemorrágica Argentina" (in Spanish). Universidad Blas Pascal. 2001. http://www.ubp.edu.ar/todoambiente/salud/enfermedades.html.
- "Fiebre hemorrágica argentina" (in Spanish). National Administration of Laboratories and Health Institutes (ANLIS). http://www.anlis.gov.ar/consulta/consulta_fharg.htm.
External links
| Classification |
|---|
 |
 KSF
KSF