Benign prostatic hyperplasia
Topic: Medicine
 From HandWiki - Reading time: 30 min
From HandWiki - Reading time: 30 min
| Benign prostatic hyperplasia | |
|---|---|
| Other names | Benign enlargement of the prostate (BEP, BPE), adenofibromyomatous hyperplasia, benign prostatic hypertrophy,[1] benign prostatic obstruction[1] |
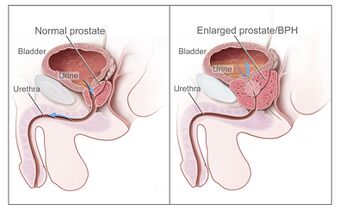 | |
| Diagram of a normal prostate (left) and benign prostatic hyperplasia (right) | |
| Specialty | Urology |
| Symptoms | Frequent urination, trouble starting to urinate, weak stream, inability to urinate, loss of bladder control[1] |
| Complications | Urinary tract infections, bladder stones, kidney failure[2] |
| Usual onset | Age over 40[1] |
| Causes | Unclear[1] |
| Risk factors | Family history, obesity, type 2 diabetes, not enough exercise, erectile dysfunction[1] |
| Diagnostic method | Based on symptoms and examination after ruling out other possible causes[2] |
| Differential diagnosis | Heart failure, diabetes, prostate cancer[2] |
| Treatment | Lifestyle changes, medications, several procedures, surgery[1][2] |
| Medication | Alpha blockers such as terazosin, 5α-reductase inhibitors such as finasteride[1] |
| Frequency | 94 million men affected globally (2019)[3] |

Benign prostatic hyperplasia (BPH), also called prostate enlargement, is a noncancerous increase in size of the prostate gland.[1] Symptoms may include frequent urination, trouble starting to urinate, weak stream, inability to urinate, or loss of bladder control.[1] Complications can include urinary tract infections, bladder stones, and chronic kidney problems.[2]
The cause is unclear.[1] Risk factors include a family history, obesity, type 2 diabetes, not enough exercise, and erectile dysfunction.[1] Medications like pseudoephedrine, anticholinergics, and calcium channel blockers may worsen symptoms.[2] The underlying mechanism involves the prostate pressing on the urethra thereby making it difficult to pass urine out of the bladder.[1] Diagnosis is typically based on symptoms and examination after ruling out other possible causes.[2]
Treatment options include lifestyle changes, medications, a number of procedures, and surgery.[1][2] In those with mild symptoms, weight loss, decreasing caffeine intake, and exercise are recommended, although the quality of the evidence for exercise is low.[2][4] In those with more significant symptoms, medications may include alpha blockers such as terazosin or 5α-reductase inhibitors such as finasteride.[1] Surgical removal of part of the prostate may be carried out in those who do not improve with other measures.[2] Some herbal medicines that have been studied, such as saw palmetto, have not been shown to help.[2] Other herbal medicines somewhat effective at improving urine flow include beta-sitosterol[5] from Hypoxis rooperi (African star grass), pygeum (extracted from the bark of Prunus africana),[6] pumpkin seeds (Cucurbita pepo), and stinging nettle (Urtica dioica) root.[7]
As of 2019[update], about 94 million men aged 40 years and older are affected globally.[3] BPH typically begins after the age of 40.[1] The prevalence of clinically diagnosed BPH peaks at 24% in men aged 75–79 years.[3] Based on autopsy studies, half of males aged 50 and over are affected, and this figure climbs to 80% after the age of 80.[3] Although prostate specific antigen levels may be elevated in males with BPH, the condition does not increase the risk of prostate cancer.[8]

Signs and symptoms
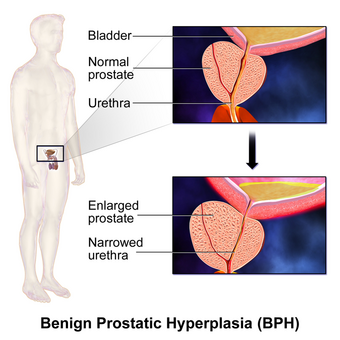
BPH is the most common cause of lower urinary tract symptoms (LUTS), which are divided into storage, voiding, and symptoms which occur after urination.[12] Storage symptoms include the need to urinate frequently, waking at night to urinate, urgency (compelling need to void that cannot be deferred), involuntary urination, including involuntary urination at night, or urge incontinence (urine leak following a strong sudden need to urinate).[13] Voiding symptoms include urinary hesitancy (a delay between trying to urinate and the flow actually beginning), intermittency (not continuous),[14] involuntary interruption of voiding, weak urinary stream, straining to void, a sensation of incomplete emptying, and uncontrollable leaking after the end of urination.[15][16][17] These symptoms may be accompanied by bladder pain or pain while urinating, called dysuria.[18]
Bladder outlet obstruction (BOO) can be caused by BPH.[19] Symptoms are abdominal pain, a continuous feeling of a full bladder, frequent urination, acute urinary retention (inability to urinate), pain during urination (dysuria), problems starting urination (urinary hesitancy), slow urine flow, starting and stopping (urinary intermittency), and nocturia.[20]
BPH can be a progressive disease, especially if left untreated. Incomplete voiding results in residual urine or urinary stasis, which can lead to an increased risk of urinary tract infection.[21]
Causes
Hormones
Most experts consider androgens (testosterone and related hormones) to play a permissive role in the development of BPH. This means that androgens must be present for BPH to occur, but do not necessarily directly cause the condition. This is supported by evidence suggesting that castrated boys do not develop BPH when they age. In a study of 26 eunuchs from the palace of the Qing dynasty still living in Beijing in 1960, the prostate could not be felt in 81% of the studied eunuchs.[22] The average time since castration was 54 years (range, 41–65 years). On the other hand, some studies suggest that administering exogenous testosterone is not associated with a significant increase in the risk of BPH symptoms, so the role of testosterone in prostate cancer and BPH is still unclear. Further randomized controlled trials with more participants are needed to quantify any risk of giving exogenous testosterone.[23]
Dihydrotestosterone (DHT), a metabolite of testosterone, is a critical mediator of prostatic growth. DHT is synthesized in the prostate from circulating testosterone by the action of the enzyme 5α-reductase, type 2. DHT can act in an autocrine fashion on the stromal cells or in paracrine fashion by diffusing into nearby epithelial cells. In both of these cell types, DHT binds to nuclear androgen receptors and signals the transcription of growth factors that are mitogenic to the epithelial and stromal cells. DHT is ten times more potent than testosterone because it dissociates from the androgen receptor more slowly. The importance of DHT in causing nodular hyperplasia is supported by clinical observations in which an inhibitor of 5α-reductase such as finasteride is given to men with this condition. Therapy with a 5α-reductase inhibitor markedly reduces the DHT content of the prostate and, in turn, reduces prostate volume and BPH symptoms.[24][25]
Testosterone promotes prostate cell proliferation,[26] but relatively low levels of serum testosterone are found in patients with BPH.[27][28] One small study has shown that medical castration lowers the serum and prostate hormone levels unevenly, having less effect on testosterone and DHT levels in the prostate.[29]
Besides testosterone and DHT, other androgens are also known to play a crucial role in BPH development. C21 11-oxygenated steroids (pregnanes) have been identified are precursors to 11-oxygenated androgens which are also potent agonists for the androgen receptor.[30] Specifically, steroids like 11β-hydroxyprogesterone and 11-ketoprogesterone can be converted to 11-ketodihydrotestosterone, an 11-oxo form of DHT with the same potency. These precursors have also been detected in tissue biopsy samples from patients with BPH, as well as in their serum levels.[31][32][33] Besides that, androgens biosynthesized via a backdoor pathway can contribute to the development of BPH.[31]
While there is some evidence that estrogen may play a role in the cause of BPH, this effect appears to be mediated mainly through local conversion of androgens to estrogen in the prostate tissue rather than a direct effect of estrogen itself.[34] In canine in vivo studies castration, which significantly reduced androgen levels but left estrogen levels unchanged, caused significant atrophy of the prostate.[35] Studies looking for a correlation between prostatic hyperplasia and serum estrogen levels in humans have generally shown none.[28][36]
In 2008, Gat et al. published evidence that BPH is caused by failure in the spermatic venous drainage system resulting in increased hydrostatic pressure and local testosterone levels elevated more than 100-fold above serum levels.[37] If confirmed, this mechanism explains why serum androgen levels do not seem to correlate with BPH and why giving exogenous testosterone would not make much difference.
Diet
Studies indicate that dietary patterns may affect the development of BPH, but further research is needed to clarify any important relationship.[38] Studies from China suggest that greater protein intake may be a factor in the development of BPH. Men older than 60 in rural areas had very low rates of clinical BPH, while men living in cities and consuming more animal protein had a higher incidence.[39][40] On the other hand, a study in Japanese-American men in Hawaii found a strong negative association with alcohol intake, but a weak positive association with beef intake.[41] In a large prospective cohort study in the US (the Health Professionals Follow-up Study), investigators reported modest associations between BPH (men with strong symptoms of BPH or surgically confirmed BPH) and total energy and protein, but not fat intake.[42] There is also epidemiological evidence linking BPH with metabolic syndrome (concurrent obesity, impaired glucose metabolism and diabetes, high triglyceride levels, high levels of low-density cholesterol, and hypertension).[43]
Degeneration
Benign prostatic hyperplasia is an age-related disease. Misrepair-accumulation aging theory[44] suggests that the development of benign prostatic hyperplasia is a consequence of fibrosis and weakening of the muscular tissue in the prostate.[45] The muscular tissue is important in the functionality of the prostate, and provides the force for excreting the fluid produced by prostatic glands. However, repeated contractions and dilations of myofibers will unavoidably cause injuries and broken myofibers. Myofibers have a low potential for regeneration; therefore, collagen fibers need to be used to replace the broken myofibers. Such misrepairs make the muscular tissue weak in functioning, and the fluid secreted by glands cannot be excreted completely. Then, the accumulation of fluid in glands increases the resistance of muscular tissue during the movements of contractions and dilations, and more and more myofibers will be broken and replaced by collagen fibers.[46]
Pathophysiology
As men age, the enzymes aromatase and 5-alpha reductase increase in activity. These enzymes are responsible for converting androgen hormones into estrogen and DHT, respectively. This metabolism of androgen hormones leads to a decrease in testosterone but increased levels of DHT and estrogen.
Both the glandular epithelial cells and the stromal cells (including muscular fibers) undergo hyperplasia in BPH.[2] Most sources agree that of the two tissues, stromal hyperplasia predominates, but the exact ratio of the two is unclear.[47]: 694
Anatomically the median and lateral lobes are usually enlarged, due to their highly glandular composition. The anterior lobe has little in the way of glandular tissue and is seldom enlarged. (Carcinoma of the prostate typically occurs in the posterior lobe – hence the ability to discern an irregular outline per rectal examination). The earliest microscopic signs of BPH usually begin between the age of 30 and 50 years old in the PUG, which is posterior to the proximal urethra.[47]: 694 In BPH, the majority of growth occurs in the transition zone (TZ) of the prostate.[47]: 694 In addition to these two classic areas, the peripheral zone (PZ) is also involved to a lesser extent.[47]: 695 Prostatic cancer typically occurs in the PZ. However, BPH nodules, usually from the TZ are often biopsied anyway to rule out cancer in the TZ.[47]: 695 BPH can be a progressive growth that in rare instances leads to exceptional enlargement.[48] In some males, the prostate enlargement exceeds 200 to 500 grams.[48] This condition has been defined as giant prostatic hyperplasia (GPH).[48]
Diagnosis
The clinical diagnosis of BPH is based on a history of LUTS (lower urinary tract symptoms), a digital rectal exam, and the exclusion of other causes of similar signs and symptoms. The degree of LUTS does not necessarily correspond to the size of the prostate. An enlarged prostate gland on rectal examination that is symmetric and smooth supports a diagnosis of BPH.[2] However, if the prostate gland feels asymmetrical, firm, or nodular, this raises concern for prostate cancer.[2]
Validated questionnaires such as the American Urological Association Symptom Index (AUA-SI), the International Prostate Symptom Score (I-PSS), and more recently the UWIN score (urgency, weak stream, incomplete emptying, and nocturia) are useful aids to making the diagnosis of BPH and quantifying the severity of symptoms.[2][49][50]
Laboratory investigations
Urinalysis is typically performed when LUTS are present and BPH is suspected to evaluate for signs of a urinary tract infection, glucose in the urine (suggestive of diabetes), or protein in the urine (suggestive of kidney disease).[2] Bloodwork including kidney function tests and prostate specific antigen (PSA) are often ordered to evaluate for kidney damage and prostate cancer, respectively.[2] However, checking blood PSA levels for prostate cancer screening is controversial and not necessarily indicated in every evaluation for BPH.[2] Benign prostatic hyperplasia and prostate cancer are both capable of increasing blood PSA levels and PSA elevation is unable to differentiate these two conditions well.[2] If PSA levels are checked and are high, then further investigation is warranted. Measures including PSA density, free PSA, rectal examination, and transrectal ultrasonography may help determine whether a PSA increase is due to BPH or prostate cancer.[2]
Imaging and other investigations
Uroflowmetry is done to measure the rate of urine flow and total volume of urine voided when the subject is urinating.[51]
Abdominal ultrasound examination of the prostate and kidneys is often performed to rule out hydronephrosis and hydroureter. Incidentally, cysts, tumours, and stones may be found on ultrasound. Post-void residual volume of more than 100 ml may indicate significant obstruction.[52] Prostate size of 30 cc or more indicates enlargement of the prostate.[53]
Prostatic calcification can be detected through transrectal ultrasound (TRUS). Calcification is due to solidification of prostatic secretions or calcified corpora amylacea (hyaline masses on the prostate gland). Calcification is also found in a variety of other conditions such as prostatitis, chronic pelvic pain syndrome, and prostate cancer.[54][55] For those with elevated levels of PSA, TRUS guided biopsy is performed to take a sample of the prostate for investigation.[56] Although MRI is more accurate than TRUS in determining prostate volume, TRUS is less expensive and almost as accurate as MRI. Therefore, TRUS is still preferred to measure prostate volume.[57]
Differential diagnosis
Medical conditions
The differential diagnosis for LUTS is broad and includes various medical conditions, neurologic disorders, and other diseases of the bladder, urethra, and prostate such as bladder cancer, urinary tract infection, urethral stricture, urethral calculi (stones), chronic prostatitis, and prostate cancer.[2] Neurogenic bladder can cause urinary retention and cause symptoms similar to those of BPH. This may occur as a result of uncoordinated contraction of the bladder muscle or impairment in the timing of bladder muscle contraction and urethral sphincter relaxation.[2] Notable causes of neurogenic bladder include disorders of the central nervous system such as Parkinson's disease, multiple sclerosis, and spinal cord injuries as well as disorders of the peripheral nervous system such as diabetes mellitus, vitamin B12 deficiency, and alcohol-induced nerve damage.[2] Individuals affected by heart failure often experience nighttime awakenings to urinate due to redistribution of fluid accumulated in swollen legs.[2]
Medications
Certain medications can increase urination difficulties by increasing bladder outlet resistance due to increased smooth muscle tone at the prostate or bladder neck and contribute to LUTS.[2] Alpha-adrenergic agonist medications, such as decongestants with pseudoephedrine can increase bladder outlet resistance.[2] In contrast, calcium channel blockers and anticholinergic medications can worsen urinary retention by promoting bladder muscle relaxation.[2] Diuretic medications such as loop diuretics (e.g., furosemide) or thiazides (e.g., chlorthalidone) can cause or worsen urinary frequency and nighttime awakenings to urinate.[2]
-
Micrograph showing nodular hyperplasia (left off center) of the prostate from a transurethral resection of the prostate (TURP). H&E stain.
-
Microscopic examination of different types of prostate tissues (stained with immunohistochemical techniques): A. Normal (non-neoplastic) prostatic tissue (NNT). B. Benign prostatic hyperplasia. C. High-grade prostatic intraepithelial neoplasia. D. Prostatic adenocarcinoma (PCA).
Management
When treating and managing benign prostatic hyperplasia, the aim is to prevent complications related to the disease and improve or relieve symptoms.[58] Approaches used include lifestyle modifications, medications, catheterization, and surgery.
Lifestyle


Lifestyle alterations to address the symptoms of BPH include physical activity,[4] decreasing fluid intake before bedtime, moderating the consumption of alcohol and caffeine-containing products, and following a timed voiding schedule.
Patients can also attempt to avoid products and medications with anticholinergic properties that may exacerbate urinary retention symptoms of BPH, including antihistamines, decongestants, opioids, and tricyclic antidepressants; however, changes in medications should be done with input from a medical professional.[59]
Physical activity
Physical activity has been recommended as a treatment for urinary tract symptoms. A 2019 Cochrane review of six studies involving 652 men assessing the effects of physical activity alone, and physical activity as a part of a self-management program, among others. However, the quality of evidence was very low and therefore it remains uncertain whether physical activity is helpful in men experiencing urinary symptoms caused by benign prostatic hyperplasia.[60]
Voiding position
Voiding position when urinating may influence urodynamic parameters (urinary flow rate, voiding time, and post-void residual volume).[61] A meta-analysis found no differences between the standing and sitting positions for healthy males, but that, for elderly males with lower urinary tract symptoms, voiding in the sitting position-- [62]
- decreased the post-void residual volume;
- increased the maximum urinary flow, comparable with pharmacological intervention; and
- decreased the voiding time.
This urodynamic profile is associated with a lower risk of urologic complications, such as cystitis and bladder stones.
Medications
The two main medication classes for BPH management are alpha blockers and 5α-reductase inhibitors.[63]
Alpha-blockers
Selective α1-blockers are the most common choice for initial therapy.[64][65][66] They include alfuzosin,[67][68] doxazosin,[69] silodosin, tamsulosin, terazosin, and naftopidil.[58] They have a small to moderate benefit at improving symptoms.[70][58][71] Selective alpha-1 blockers are similar in effectiveness but have slightly different side effect profiles.[70][58][71] Alpha blockers relax smooth muscle in the prostate and the bladder neck, thus decreasing the blockage of urine flow. Common side effects of alpha-blockers include orthostatic hypotension (a head rush or dizzy spell when standing up or stretching), ejaculation changes, erectile dysfunction,[72] headaches, nasal congestion, and weakness. For men with LUTS due to an enlarged prostate, the effects of naftopidil, tamsulosin, and silodosin on urinary symptoms and quality of life may be similar.[58] Naftopidil and tamsulosin may have similar levels of unwanted sexual side effects but fewer unwanted side effects than silodosin.[58]
Tamsulosin and silodosin are selective α1 receptor blockers that preferentially bind to the α1A receptor in the prostate instead of the α1B receptor in the blood vessels. Less-selective α1 receptor blockers such as terazosin and doxazosin may lower blood pressure. The older, less selective α1-adrenergic blocker prazosin is not a first-line choice for either high blood pressure or prostatic hyperplasia; it is a choice for patients who present with both problems at the same time. The older, broadly non-selective alpha-blocker medications such as phenoxybenzamine are not recommended for control of BPH.[73] Non-selective alpha-blockers such as terazosin and doxazosin may also require slow dose adjustments as they can lower blood pressure and cause syncope (fainting) if the response to the medication is too strong.
5α-reductase inhibitors
The 5α-reductase inhibitors finasteride and dutasteride may also be used in people with BPH.[74] These medications inhibit the 5α-reductase enzyme, which, in turn, inhibits the production of DHT, a hormone responsible for enlarging the prostate. Effects may take longer to appear than alpha blockers, but they persist for many years.[75] When used together with alpha-blockers, no benefit was reported in short-term trials, but in a longer-term study (3–4 years) there was a greater reduction in BPH progression to acute urinary retention and surgery than with either agent alone, especially in people with more severe symptoms and larger prostates.[76][77][78] Other trials have confirmed reductions in symptoms, within 6 months in one trial, an effect that was maintained after withdrawal of the alpha blocker.[77][79] Side effects include decreased libido and ejaculatory or erectile dysfunction.[80][81] The 5α-reductase inhibitors are contraindicated in pregnant women because of their teratogenicity due to interference with fetal testosterone metabolism, and as a precaution, pregnant women should not handle crushed or broken tablets.[82]


Phosphodiesterase inhibitors (PDE)
A 2018 Cochrane review of studies on men over 60 with moderate to severe lower urinary tract symptoms analyzed the impacts of phosphodiesterase inhibitors (PDE) in comparison to other drugs.[89] These drugs may improve urinary symptoms slightly and reduce urinary bother but may also cause more side effects than placebo. The evidence in this review found that there is probably no difference between PDE and alpha blockers, however when used in combination they may provide a greater improvement in symptoms (with more side effects). PDE also likely improves symptoms when used with 5-alpha reductase inhibitors.
Several phosphodiesterase-5 inhibitors are also effective but may require multiple doses daily to maintain adequate urine flow.[90][91] Tadalafil, a phosphodiesterase-5 inhibitor, was considered then rejected by NICE in the UK for the treatment of symptoms associated with BPH.[92] In 2011, the U.S. Food and Drug Administration approved tadalafil to treat the signs and symptoms of benign prostatic hyperplasia, and for the treatment of BPH and erectile dysfunction (ED), when the conditions occur simultaneously.[93]
Others
Antimuscarinics such as tolterodine may also be used, especially in combination with alpha-blockers.[94] They act by decreasing acetylcholine effects on the smooth muscle of the bladder, thus helping control symptoms of an overactive bladder.[95]
Self-catheterization
Intermittent urinary catheterization is used to relieve the bladder in people with urinary retention. Self-catheterization is an option in BPH when it is difficult or impossible to empty the bladder.[96] Urinary tract infection is the most common complication of intermittent catheterization.[97] Several techniques and types of catheter are available, including sterile (single-use) and clean (multiple use) catheters, but, based on current information, none is superior to others in reducing the incidence of urinary tract infection.[98]
Surgery

If medical treatment is not effective, surgery may be performed. Surgical techniques used include the following:
- Transurethral resection of the prostate (TURP): the gold standard.[99] TURP is thought to be the most effective approach for improving urinary symptoms and urinary flow, however, this surgical procedure may be associated with complications in up to 20% of men.[99] Surgery carries some risk of complications, such as retrograde ejaculation (most commonly), erectile dysfunction, urinary incontinence, urethral strictures.[100]
- Transurethral incision of the prostate (TUIP): rarely performed; the technique is similar to TURP but less definitive.
- Open prostatectomy: not usually performed nowadays due to its high morbidity, even if the results are excellent.
Other less invasive surgical approaches (requiring spinal anesthesia) include:
- Holmium laser ablation of the prostate (HoLAP)
- Holmium laser enucleation of the prostate (HoLeP)
- Thulium laser transurethral vaporesection of the prostate (ThuVARP)
- Photoselective vaporization of the prostate (PVP)
- Aquablation therapy: a type of surgery using a water jet to remove prostatic tissue.
Minimally invasive procedures
Some less invasive procedures are available according to patients' preferences and co-morbidities. These are performed as outpatient procedures with local anesthesia.
- Prostatic artery embolization: an endovascular procedure performed in interventional radiology.[101] Through catheters, embolic agents are released in the main branches of the prostatic artery, in order to induce a decrease in the size of the prostate gland, thus reducing the urinary symptoms.[102]
- Water vapor thermal therapy (marketed as Rezum): This is a newer office procedure for removing prostate tissue using steam aimed at preserving sexual function.
- Prostatic urethral lift (marketed as UroLift): This intervention consists of a system of a device and an implant designed to pull the prostatic lobe away from the urethra.[103]
- Transurethral microwave thermotherapy (TUMT) is an outpatient procedure that is less invasive compared to surgery and involves using microwaves (heat) to shrink prostate tissue that is enlarged.[99]
- Temporary implantable nitinol device (TIND and iTIND): is a device that is placed in the urethra that, when released, is expanded, reshaping the urethra and the bladder neck.[104]



Alternative medicine
While herbal remedies are commonly used, a 2016 review found the herbs studied to be no better than placebos.[154] Particularly, several reviews found that saw palmetto extract, while one of the most commonly used, is no better than a placebo both in symptom relief and in decreasing prostate size.[155][156][157]
Epidemiology

Globally, benign prostatic hyperplasia affects about 94 million males as of 2019[update].[3]
The prostate gets larger in most men as they get older. For a symptom-free man of 46 years, the risk of developing BPH over the next 30 years is 45%. Incidence rates increase from 3 cases per 1000 man-years at age 45–49 years, to 38 cases per 1000 man-years by the age of 75–79 years. While the prevalence rate is 2.7% for men aged 45–49, it increases to 24% by the age of 80 years.[159]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 "Prostate Enlargement (Benign Prostatic Hyperplasia)". September 2014. https://www.niddk.nih.gov/health-information/urologic-diseases/prostate-problems/enlarged-prostate-benign-prostatic-hyperplasia?dkrd=/health-information/urologic-diseases/prostate-problems/prostate-enlargement-benign-prostatic-hyperplasia.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 2.26 2.27 "Management of Benign Prostatic Hyperplasia". Annual Review of Medicine 67: 137–151. 2016. doi:10.1146/annurev-med-063014-123902. PMID 26331999.
- ↑ 3.0 3.1 3.2 3.3 3.4 "The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the Global Burden of Disease Study 2019". The Lancet. Healthy Longevity 3 (11): e754–e776. November 2022. doi:10.1016/S2666-7568(22)00213-6. PMID 36273485.
- ↑ 4.0 4.1 "Physical activity for lower urinary tract symptoms secondary to benign prostatic obstruction". The Cochrane Database of Systematic Reviews 2019 (4). April 2019. doi:10.1002/14651858.CD012044.pub2. PMID 30953341.
- ↑ "Beta-sitosterols for benign prostatic hyperplasia". The Cochrane Database of Systematic Reviews 1999 (2). 1999. doi:10.1002/14651858.CD001043. PMID 10796740.
- ↑ "Pygeum africanum for benign prostatic hyperplasia". The Cochrane Database of Systematic Reviews 1998 (1). 1998. doi:10.1002/14651858.CD001044. PMID 11869585.
- ↑ "Phytotherapy for benign prostatic hyperplasia". Public Health Nutrition 3 (4A): 459–472. December 2000. doi:10.1017/S1368980000000549. PMID 11276294.
- ↑ "Is there a link between BPH and prostate cancer?". The Practitioner 256 (1750): 13–6, 2. April 2012. PMID 22792684.
- ↑ "The development of human benign prostatic hyperplasia with age". The Journal of Urology 132 (3): 474–479. September 1984. doi:10.1016/S0022-5347(17)49698-4. PMID 6206240.
- ↑ "The prevalence of prostatism: a population-based survey of urinary symptoms". The Journal of Urology 150 (1): 85–89. July 1993. doi:10.1016/S0022-5347(17)35405-8. PMID 7685427.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 "NHS England » Decision support tool: making a decision about enlarged prostate (BPE)". 21 November 2023. https://www.england.nhs.uk/publication/decision-support-tool-making-a-decision-about-enlarged-prostate-bpe/.
- ↑ Lower urinary tract symptoms in men: management, NICE (National Institute for Health and Care Excellence)
- ↑ "Urge incontinence". US National Library of Medicine. https://www.medlineplus.gov/ency/article/001270.htm.
- ↑ "Incontinence and Stream Abnormalities". Clinical Methods: The History, Physical, and Laboratory Examinations (3rd ed.). Boston: Butterworths. 1990. ISBN 978-0-409-90077-4. https://www.ncbi.nlm.nih.gov/books/NBK295/.
- ↑ "Post-micturition dribble in men: causes and treatment". Nursing Standard 22 (30): 43–46. 11 February 2008. doi:10.7748/ns2008.04.22.30.43.c6440. PMID 18459613.
- ↑ "Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms". The New England Journal of Medicine 367 (3): 248–257. July 2012. doi:10.1056/nejmcp1106637. PMID 22808960.
- ↑ "Urination – difficulty with flow". US National Library of Medicine. https://www.medlineplus.gov/ency/article/003143.htm.
- ↑ "Urination – painful". US National Library of Medicine. https://www.medlineplus.gov/ency/article/003145.htm.
- ↑ "Bladder outlet obstruction". US National Library of Medicine. https://www.medlineplus.gov/ency/article/002238.htm.
- ↑ "Benign Prostatic Hyperplasia". https://www.lecturio.com/concepts/benign-prostatic-hyperplasia/.
- ↑ "Residual urinary volume and urinary tract infection--when are they linked?". The Journal of Urology 180 (1): 182–185. July 2008. doi:10.1016/j.juro.2008.03.044. PMID 18499191.
- ↑ "The prostate in eunuchs". Progress in Clinical and Biological Research 370: 249–255. 1991. PMID 1924456.
- ↑ "Testosterone and Aging: Clinical Research Directions.". NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK216175/.
- ↑ "Proscar (finasteride) Prescribing Information". Merck and Company. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020180s037lbl.pdf.
- ↑ "Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia". World Journal of Urology 19 (6): 413–425. April 2002. doi:10.1007/s00345-002-0248-5. PMID 12022710.
- ↑ "The development of androgen-independent prostate cancer". Nature Reviews. Cancer 1 (1): 34–45. October 2001. doi:10.1038/35094009. PMID 11900250.
- ↑ "Serum steroids in relation to benign prostatic hyperplasia". Oncology 54 (6): 497–501. 1997. doi:10.1159/000227609. PMID 9394847.
- ↑ 28.0 28.1 "Serum sex hormones and measures of benign prostatic hyperplasia". The Prostate 61 (2): 124–131. October 2004. doi:10.1002/pros.20080. PMID 15305335.
- ↑ "Persistent intraprostatic androgen concentrations after medical castration in healthy men". The Journal of Clinical Endocrinology and Metabolism 91 (10): 3850–3856. October 2006. doi:10.1210/jc.2006-0968. PMID 16882745.
- ↑ "Adrenocortical hormone abnormalities in men with chronic prostatitis/chronic pelvic pain syndrome". Urology 71 (2): 261–266. February 2008. doi:10.1016/j.urology.2007.09.025. PMID 18308097.
- ↑ 31.0 31.1 "The 11β-hydroxyandrostenedione pathway and C11-oxy C21 backdoor pathway are active in benign prostatic hyperplasia yielding 11keto-testosterone and 11keto-progesterone". The Journal of Steroid Biochemistry and Molecular Biology 196. February 2020. doi:10.1016/j.jsbmb.2019.105497. PMID 31626910.
- ↑ ""Re: Adrenocortical Hormone Abnormalities in Men With Chronic Prostatitis/Chronic Pelvic Pain Syndrome"" (in English). Urology 169: 273. November 2022. doi:10.1016/j.urology.2022.07.051. PMID 35987379.
- ↑ "Author Reply" (in English). Urology 169: 273–274. November 2022. doi:10.1016/j.urology.2022.07.049. PMID 35985522.
- ↑ "Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen". The Journal of Endocrinology 197 (3): 483–491. June 2008. doi:10.1677/JOE-07-0470. PMID 18492814.
- ↑ "Androgen and prostatic stroma". Asian Journal of Andrology 5 (1): 19–26. March 2003. PMID 12646998.
- ↑ "Serum sex steroids, gonadotrophins and sex hormone-binding globulin in prostatic hyperplasia". Annals of Saudi Medicine 28 (3): 174–178. 2008. doi:10.4103/0256-4947.51727. PMID 18500180.
- ↑ "Reversal of benign prostate hyperplasia by selective occlusion of impaired venous drainage in the male reproductive system: novel mechanism, new treatment". Andrologia 40 (5): 273–281. October 2008. doi:10.1111/j.1439-0272.2008.00883.x. PMID 18811916.
- ↑ "Prostate enlargement: the canary in the coal mine?". The American Journal of Clinical Nutrition 75 (4): 605–606. April 2002. doi:10.1093/ajcn/75.4.605. PMID 11916745.
- ↑ "[Comparison of incidence of BPH and related factors between urban and rural inhabitants in district of Wannan]". Zhonghua Nan Ke Xue = National Journal of Andrology 9 (1): 45–47. February 2003. PMID 12680332.
- ↑ "Changes in the prevalence of benign prostatic hyperplasia in China". Chinese Medical Journal 110 (3): 163–166. March 1997. PMID 9594331.
- ↑ "A prospective study of alcohol, diet, and other lifestyle factors in relation to obstructive uropathy". The Prostate 22 (3): 253–264. 1993. doi:10.1002/pros.2990220308. PMID 7683816.
- ↑ "Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia". The American Journal of Clinical Nutrition 75 (4): 689–697. April 2002. doi:10.1093/ajcn/75.4.689. PMID 11916755.
- ↑ "Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis". BJU International 115 (1): 24–31. January 2015. doi:10.1111/bju.12728. PMID 24602293.
- ↑ "Aging as a consequence of misrepair--A novel theory of aging.". Nature Precedings. March 2009. doi:10.1038/npre.2009.2988.1.
- ↑ Wang-Michelitsch J, Michelitsch T (2015). "Tissue fibrosis: a principal evidence for the central role of Misrepairs in aging". arXiv:1503.01376 [cs.DM].
- ↑ "Benign prostatic hyperplasia: an overview". Reviews in Urology 7 (Suppl 9): S3–S14. 2005. PMID 16985902.
- ↑ 47.0 47.1 47.2 47.3 47.4 "Benign prostatic hyperplasia: a review and ultrasound classification". Radiologic Clinics of North America 44 (5): 689–710, viii. September 2006. doi:10.1016/j.rcl.2006.07.005. PMID 17030221.
- ↑ 48.0 48.1 48.2 "Management of a giant prostatic enlargement: Case report and review of the literature". The Nigerian Postgraduate Medical Journal (Medknow) 27 (3): 242–247. 2020. doi:10.4103/npmj.npmj_69_20. PMID 32687126.
- ↑ "Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors". Current Bladder Dysfunction Reports 5 (4): 212–218. December 2010. doi:10.1007/s11884-010-0067-2. PMID 21475707.
- ↑ "Validation of the Urgency, Weak stream, Incomplete emptying, and Nocturia (UWIN) score compared with the American Urological Association Symptoms Score in assessing lower urinary tract symptoms in the clinical setting". Urology 83 (1): 181–185. January 2014. doi:10.1016/j.urology.2013.08.039. PMID 24139351.
- ↑ "The fundamentals of uroflowmetry practice, based on International Continence Society good urodynamic practices recommendations". Neurourology and Urodynamics 37 (S6): S44–S49. August 2018. doi:10.1002/nau.23777. PMID 30614059.
- ↑ "The Role of Transabdominal Ultrasound in Office Urology" (in en). Proceedings of Singapore Healthcare 22 (2): 125–130. June 2013. doi:10.1177/201010581302200208. ISSN 2010-1058.
- ↑ "Improving ultrasound-based prostate volume estimation". BMC Urology 19 (1). July 2019. doi:10.1186/s12894-019-0492-2. PMID 31340802.
- ↑ "Benign Conditions That Mimic Prostate Carcinoma: MR Imaging Features with Histopathologic Correlation". Radiographics 36 (1): 162–175. January 2016. doi:10.1148/rg.2016150030. PMID 26587887.
- ↑ "Prostatic calcifications: Quantifying occurrence, radiodensity, and spatial distribution in prostate cancer patients". Urologic Oncology 39 (10): 728.e1–728.e6. October 2021. doi:10.1016/j.urolonc.2020.12.028. PMID 33485763.
- ↑ "Ultrasound of the prostate". Cancer Imaging 10 (1): 40–48. March 2010. doi:10.1102/1470-7330.2010.0004. PMID 20199941.
- ↑ "Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostate volume as compared with radical prostatectomy specimens". Urologia Internationalis 78 (4): 323–327. 2007. doi:10.1159/000100836. PMID 17495490. http://www.karger.com/Article/FullText/100836.
- ↑ 58.0 58.1 58.2 58.3 58.4 58.5 "Naftopidil for the treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia". The Cochrane Database of Systematic Reviews 2018 (10). October 2018. doi:10.1002/14651858.CD007360.pub3. PMID 30306544.
- ↑ "Benign prostatic hyperplasia". University of Maryland Medical Center. http://umm.edu/health/medical/reports/articles/benign-prostatic-hyperplasia.
- ↑ "Physical activity for lower urinary tract symptoms secondary to benign prostatic obstruction". The Cochrane Database of Systematic Reviews 2019 (4). April 2019. doi:10.1002/14651858.CD012044.pub2. PMID 30953341.
- ↑ "Influence of voiding posture on urodynamic parameters in men: a literature review". Nederlands Tijdschrift voor urologie. http://www.mednet.nl/wosmedia/1718/mictiehouding_tvu.pdf.
- ↑ "Urinating standing versus sitting: position is of influence in men with prostate enlargement. A systematic review and meta-analysis". PLOS ONE 9 (7). 2014. doi:10.1371/journal.pone.0101320. PMID 25051345. Bibcode: 2014PLoSO...9j1320D.
- ↑ "Current medical treatment of lower urinary tract symptoms/BPH: do we have a standard?". Current Opinion in Urology 24 (1): 21–28. January 2014. doi:10.1097/mou.0000000000000007. PMID 24231531.
- ↑ "The benign prostatic hyperplasia registry and patient survey: study design, methods, and patient baseline characteristics". BJU International 100 (4): 813–819. October 2007. doi:10.1111/j.1464-410X.2007.07061.x. PMID 17822462.
- ↑ "An examination of treatment patterns and costs of care among patients with benign prostatic hyperplasia". The American Journal of Managed Care 12 (4 Suppl): S99–S110. March 2006. PMID 16551208. http://www.ajmc.com/pubMed.php?pii=3096.
- ↑ "The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries". European Urology 51 (1): 207–15; discussion 215–6. January 2007. doi:10.1016/j.eururo.2006.06.012. PMID 16846678.
- ↑ "Alfuzosin for treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia: a systematic review of efficacy and adverse effects". Urology 66 (4): 780–788. October 2005. doi:10.1016/j.urology.2005.05.001. PMID 16230138.
- ↑ "Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial". Urology 58 (6): 953–959. December 2001. doi:10.1016/S0090-4295(01)01448-0. PMID 11744466.
- ↑ "Doxazosin for treating lower urinary tract symptoms compatible with benign prostatic obstruction: a systematic review of efficacy and adverse effects". BJU International 94 (9): 1263–1270. December 2004. doi:10.1111/j.1464-410X.2004.05154.x. PMID 15610102.
- ↑ 70.0 70.1 "Tamsulosin for benign prostatic hyperplasia". The Cochrane Database of Systematic Reviews (1). 2003. doi:10.1002/14651858.CD002081. PMID 12535426.
- ↑ 71.0 71.1 "A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction". European Urology 36 (1): 1–13. 1999. doi:10.1159/000019919. PMID 10364649.
- ↑ "Treatment of benign prostatic hyperplasia in patients with cardiovascular disease". Drugs & Aging 23 (10): 795–805. 2006. doi:10.2165/00002512-200623100-00003. PMID 17067183.
- ↑ AUA Practice Guidelines Committee (August 2003). "AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations". The Journal of Urology 170 (2 Pt 1): 530–547. doi:10.1097/01.ju.0000078083.38675.79. PMID 12853821.
- ↑ "BPH update: medical versus interventional management". The Canadian Journal of Urology 23 (Suppl 1): 10–15. February 2016. PMID 26924590. http://www.canjurol.com/html/free-articles/V23I1S1F-07_DrElterman.pdf.
- ↑ "Sustained decrease in incidence of acute urinary retention and surgery with finasteride for 6 years in men with benign prostatic hyperplasia". The Journal of Urology 171 (3): 1194–1198. March 2004. doi:10.1097/01.ju.0000112918.74410.94. PMID 14767299.
- ↑ "Influence of baseline variables on changes in International Prostate Symptom Score after combined therapy with dutasteride plus tamsulosin or either monotherapy in patients with benign prostatic hyperplasia and lower urinary tract symptoms: 4-year results of the CombAT study". BJU International 113 (4): 623–635. April 2014. doi:10.1111/bju.12500. PMID 24127818.
- ↑ 77.0 77.1 "The role of combination medical therapy in benign prostatic hyperplasia". International Journal of Impotence Research 20 (Suppl 3): S33–S43. December 2008. doi:10.1038/ijir.2008.51. PMID 19002123.
- ↑ "Combination therapy with doxazosin and finasteride for benign prostatic hyperplasia in patients with lower urinary tract symptoms and a baseline total prostate volume of 25 ml or greater". The Journal of Urology 175 (1): 217–20; discussion 220–1. January 2006. doi:10.1016/S0022-5347(05)00041-8. PMID 16406915.
- ↑ "Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasteride". European Urology 44 (4): 461–466. October 2003. doi:10.1016/s0302-2838(03)00367-1. PMID 14499682.
- ↑ "The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group". The New England Journal of Medicine 327 (17): 1185–1191. October 1992. doi:10.1056/NEJM199210223271701. PMID 1383816.
- ↑ "Impact of medical treatments for male lower urinary tract symptoms due to benign prostatic hyperplasia on ejaculatory function: a systematic review and meta-analysis". The Journal of Sexual Medicine 11 (6): 1554–1566. June 2014. doi:10.1111/jsm.12525. PMID 24708055.
- ↑ "Benign Prostatic Hypertrophy Treatment & Management". http://emedicine.medscape.com/article/437359-treatment.
- ↑ 83.0 83.1 "The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia". The New England Journal of Medicine 349 (25): 2387–2398. December 2003. doi:10.1056/NEJMoa030656. PMID 14681504.
- ↑ 84.0 84.1 "The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study". European Urology 57 (1): 123–131. January 2010. doi:10.1016/j.eururo.2009.09.035. PMID 19825505.
- ↑ 85.0 85.1 "Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial". JAMA 296 (19): 2319–2328. November 2006. doi:10.1001/jama.296.19.2319. PMID 17105794.
- ↑ "Effects of alpha(1)-adrenoceptor antagonists on male sexual function". Drugs 66 (3): 287–301. 2006-02-01. doi:10.2165/00003495-200666030-00002. PMID 16526818.
- ↑ "[Negative effects on sexual function of medications for the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia]". Progres en Urologie 25 (3): 115–127. March 2015. doi:10.1016/j.purol.2014.12.003. PMID 25605342.
- ↑ 88.0 88.1 "Evidence | Lower urinary tract symptoms in men: management | Guidance | NICE". 2010-05-23. https://www.nice.org.uk/guidance/cg97/evidence.
- ↑ "Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia". The Cochrane Database of Systematic Reviews 2018 (11). November 2018. doi:10.1002/14651858.CD010060.pub2. PMID 30480763.
- ↑ "Tadalafil 5 mg Once Daily Improves Lower Urinary Tract Symptoms and Erectile Dysfunction: A Systematic Review and Meta-analysis". Lower Urinary Tract Symptoms 10 (1): 84–92. January 2018. doi:10.1111/luts.12144. PMID 29341503.
- ↑ "Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia". The Cochrane Database of Systematic Reviews 2018 (11). November 2018. doi:10.1002/14651858.CD010060.pub2. PMID 30480763.
- ↑ "Hyperplasia (benign prostatic) – tadalafil (terminated appraisal) (TA273)". National Institute for Health and Clinical Excellence (NICE). 23 January 2013. http://guidance.nice.org.uk/TA273.
- ↑ "FDA approves Cialis to treat benign prostatic hyperplasia". U.S. Food and Drug Administration (FDA). https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm274642.htm.
- ↑ "Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial". JAMA 296 (19): 2319–2328. November 2006. doi:10.1001/jama.296.19.2319. PMID 17105794.
- ↑ "Muscarinic receptor antagonists for overactive bladder". BJU International 100 (5): 987–1006. November 2007. doi:10.1111/j.1464-410x.2007.07205.x. PMID 17922784.
- ↑ "Prostate enlargement (benign prostatic hyperplasia)". Harvard Health Publications. http://www.harvardhealthcontent.com/SpecialHealthReports/70,PA0212?Page=Section9.
- ↑ "Complications of intermittent catheterization: their prevention and treatment". Spinal Cord 40 (10): 536–541. October 2002. doi:10.1038/sj.sc.3101348. PMID 12235537.
- ↑ "Intermittent catheter techniques, strategies and designs for managing long-term bladder conditions". The Cochrane Database of Systematic Reviews 10 (10). October 2021. doi:10.1002/14651858.CD006008.pub5. PMID 34699062.
- ↑ 99.0 99.1 99.2 "Transurethral microwave thermotherapy for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia". The Cochrane Database of Systematic Reviews 2021 (6). June 2021. doi:10.1002/14651858.CD004135.pub4. PMID 34180047.
- ↑ "Transurethral resection of the prostate (TURP) - Risks" (in en). 2017-10-24. https://www.nhs.uk/conditions/transurethral-resection-of-the-prostate-turp/risks/.
- ↑ "A Systematic Review of Prostatic Artery Embolization in the Treatment of Symptomatic Benign Prostatic Hyperplasia". CardioVascular and Interventional Radiology 40 (5): 655–663. May 2017. doi:10.1007/s00270-016-1539-3. PMID 28032133.
- ↑ "Prostate Embolization as an Alternative to Open Surgery in Patients with Large Prostate and Moderate to Severe Lower Urinary Tract Symptoms". Journal of Vascular and Interventional Radiology 27 (5): 700–708. May 2016. doi:10.1016/j.jvir.2016.01.138. PMID 27019980.
- ↑ "Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system". Medical Devices: Evidence and Research 9: 115–123. May 2016. doi:10.2147/MDER.S60780. PMID 27274321.
- ↑ "Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at 1 year of follow-up". BJU International 116 (2): 278–287. August 2015. doi:10.1111/bju.12982. PMID 25382816.
- ↑ 105.0 105.1 "Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study". BJU International 119 (5): 767–775. May 2017. doi:10.1111/bju.13714. PMID 27862831.
- ↑ 106.0 106.1 "Randomized Controlled Trial of Aquablation versus Transurethral Resection of the Prostate in Benign Prostatic Hyperplasia: One-year Outcomes". Urology 125: 169–173. March 2019. doi:10.1016/j.urology.2018.12.002. PMID 30552937.
- ↑ "Benign Prostatic Hyperplasia: Surgical Therapy & New Technology II (MP09)" (in en). Journal of Urology 206 (Supplement 3). September 2021. doi:10.1097/JU.0000000000001982. ISSN 0022-5347. http://www.auajournals.org/doi/10.1097/JU.0000000000001982.
- ↑ "120W GreenLight High Performance System laser for benign prostate hyperplasia: 68 patients with 3-year follow-up and analysis of predictors of response". Urology 80 (2): 396–401. August 2012. doi:10.1016/j.urology.2012.01.063. PMID 22857762.
- ↑ "Minimally invasive prostatic urethral lift (PUL) efficacious in TURP candidates: a multicenter German evaluation after 2 years". World Journal of Urology 37 (7): 1353–1360. July 2019. doi:10.1007/s00345-018-2494-1. PMID 30283994.
- ↑ 110.0 110.1 "Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study". European Urology 68 (4): 643–652. October 2015. doi:10.1016/j.eururo.2015.04.024. PMID 25937539.
- ↑ 111.0 111.1 "Minimally Invasive Prostate Convective Water Vapor Energy Ablation: A Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia". The Journal of Urology 195 (5): 1529–1538. May 2016. doi:10.1016/j.juro.2015.10.181. PMID 26614889.
- ↑ "Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezūm system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia" (in English). Research and Reports in Urology 9: 159–168. 2017-08-21. doi:10.2147/RRU.S143679. PMID 28861405.
- ↑ 113.0 113.1 "Composite urinary and sexual outcomes after Rezum: an analysis of predictive factors from an Italian multi-centric study". Prostate Cancer and Prostatic Diseases 26 (2): 410–414. June 2023. doi:10.1038/s41391-022-00587-6. PMID 36042295.
- ↑ 114.0 114.1 "Long-Term Outcome of Prostatic Artery Embolization for Patients with Benign Prostatic Hyperplasia: Single-Centre Retrospective Study in 1072 Patients Over a 10-Year Period". CardioVascular and Interventional Radiology 45 (9): 1324–1336. September 2022. doi:10.1007/s00270-022-03199-8. PMID 35778579.
- ↑ 115.0 115.1 "Transurethral Resection of the Prostate (TURP) Versus Original and PErFecTED Prostate Artery Embolization (PAE) Due to Benign Prostatic Hyperplasia (BPH): Preliminary Results of a Single Center, Prospective, Urodynamic-Controlled Analysis". CardioVascular and Interventional Radiology 39 (1): 44–52. January 2016. doi:10.1007/s00270-015-1202-4. PMID 26506952.
- ↑ 116.0 116.1 "The iTind Temporarily Implanted Nitinol Device for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled Trial". Urology 153: 270–276. July 2021. doi:10.1016/j.urology.2020.12.022. PMID 33373708.
- ↑ 117.0 117.1 "Complications of laser prostatectomy: a review of recent data". World Journal of Urology 28 (1): 53–62. February 2010. doi:10.1007/s00345-009-0504-z. PMID 20052586.
- ↑ "Comparison of Long-term Effect and Complications Between Holmium Laser Enucleation and Transurethral Resection of Prostate: Nations-Wide Health Insurance Study". Urology 154: 300–307. August 2021. doi:10.1016/j.urology.2021.04.019. PMID 33933503.
- ↑ "GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia: a randomized clinical trial with midterm follow-up". European Urology 58 (3): 349–355. September 2010. doi:10.1016/j.eururo.2010.05.026. PMID 20605316.
- ↑ 120.0 120.1 "A Multicenter Randomized Noninferiority Trial Comparing GreenLight-XPS Laser Vaporization of the Prostate and Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Obstruction: Two-yr Outcomes of the GOLIATH Study". European Urology 69 (1): 94–102. January 2016. doi:10.1016/j.eururo.2015.07.054. PMID 26283011.
- ↑ 121.0 121.1 "Global Greenlight Group: largest international Greenlight experience for benign prostatic hyperplasia to assess efficacy and safety". World Journal of Urology 39 (12): 4389–4395. December 2021. doi:10.1007/s00345-021-03688-4. PMID 33837819.
- ↑ "Vaporize, anatomically vaporize or enucleate the prostate? The flexible use of the GreenLight laser". International Urology and Nephrology 49 (3): 405–411. March 2017. doi:10.1007/s11255-016-1494-6. PMID 28044238.
- ↑ "Long-term Patient-reported Clinical Outcomes and Reoperation Rate after Photovaporization with the XPS-180W GreenLight Laser". European Urology Focus 5 (4): 676–680. July 2019. doi:10.1016/j.euf.2017.10.006. PMID 29102672. https://hal.archives-ouvertes.fr/hal-03488114/file/S2405456917302432.pdf.
- ↑ "Photoselective vaporization of the prostate with the 180-W XPS-Greenlight laser: Five-year experience of safety, efficiency, and functional outcomes". Canadian Urological Association Journal 12 (7): E318–E324. July 2018. doi:10.5489/cuaj.4895. PMID 29603912.
- ↑ "MP51-13 Four-Year Rezum Outcomes in Relationship to the Number of Injections: Is the "Less Is More" Treatment Approach Durable?" (in en). Journal of Urology 209 (Supplement 4). April 2023. doi:10.1097/JU.0000000000003299.13. ISSN 0022-5347. http://www.auajournals.org/doi/10.1097/JU.0000000000003299.13.
- ↑ 126.0 126.1 "Prostatic Urethral Lift: A Unique Minimally Invasive Surgical Treatment of Male Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia". The Urologic Clinics of North America. Treatment of Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia 43 (3): 357–369. August 2016. doi:10.1016/j.ucl.2016.04.008. PMID 27476128.
- ↑ "Minimally invasive prostatic urethral lift: surgical technique and multinational experience". European Urology 64 (2): 292–299. August 2013. doi:10.1016/j.eururo.2013.01.008. PMID 23357348.
- ↑ "Predictors of reoperation after transurethral resection of the prostate in a diverse, urban academic centre" (in en). Journal of Clinical Urology 17 (3): 238–245. May 2024. doi:10.1177/20514158221132102. ISSN 2051-4158. https://journals.sagepub.com/doi/10.1177/20514158221132102.
- ↑ "The 80-W KTP GreenLight laser vaporization of the prostate versus transurethral resection of the prostate (TURP): adjusted analysis of 5-year results of a prospective non-randomized bi-center study". Lasers in Medical Science 30 (3): 1147–1151. April 2015. doi:10.1007/s10103-015-1721-x. PMID 25698433.
- ↑ 130.0 130.1 "Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study)". BJU International 122 (2): 270–282. August 2018. doi:10.1111/bju.14249. PMID 29645352.
- ↑ 131.0 131.1 "Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: results of short- and mid-term follow-up". European Radiology 23 (9): 2561–2572. September 2013. doi:10.1007/s00330-012-2714-9. PMID 23370938.
- ↑ "UroLift for Treating Lower Urinary Tract Symptoms of Benign Prostatic Hyperplasia: A NICE Medical Technology Guidance Update". Applied Health Economics and Health Policy 20 (5): 669–680. September 2022. doi:10.1007/s40258-022-00735-y. PMID 35843995.
- ↑ "Anterograde ejaculation preservation after endoscopic treatments in patients with bladder outlet obstruction: systematic review and pooled-analysis of randomized clinical trials". Minerva Urologica e Nefrologica = The Italian Journal of Urology and Nephrology 71 (5): 427–434. October 2019. doi:10.23736/s0393-2249.19.03588-4. PMID 31487977.
- ↑ "A Systematic Review of Reported Ejaculatory Dysfunction in Clinical Trials Evaluating Minimally Invasive Treatment Modalities for BPH". Current Urology Reports 21 (12). October 2020. doi:10.1007/s11934-020-01012-y. PMID 33104947.
- ↑ "Preservation of antegrade ejaculation after surgical relief of benign prostatic obstruction is a valid endpoint". World Journal of Urology 39 (7): 2277–2289. July 2021. doi:10.1007/s00345-021-03682-w. PMID 33796882.
- ↑ "Transurethral holmium laser enucleation of the prostate versus transurethral electrocautery resection of the prostate: a randomized prospective trial in 200 patients". The Journal of Urology 172 (3): 1012–1016. September 2004. doi:10.1097/01.ju.0000136218.11998.9e. PMID 15311026.
- ↑ "GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: a randomized clinical trial with 2-year follow-up". European Urology 60 (4): 734–739. October 2011. doi:10.1016/j.eururo.2011.05.043. PMID 21658839.
- ↑ "A randomized trial comparing bipolar transurethral vaporization of the prostate with GreenLight laser (xps-180watt) photoselective vaporization of the prostate for treatment of small to moderate benign prostatic obstruction: outcomes after 2 years". BJU International 125 (1): 144–152. January 2020. doi:10.1111/bju.14926. PMID 31621175.
- ↑ "Experience with more than 1,000 holmium laser prostate enucleations for benign prostatic hyperplasia". The Journal of Urology 183 (3): 1105–1109. March 2010. doi:10.1016/j.juro.2009.11.034. PMID 20092844.
- ↑ "Randomised trial of bipolar resection vs holmium laser enucleation vs Greenlight laser vapo-enucleation of the prostate for treatment of large benign prostate obstruction: 3-years outcomes". BJU International 126 (6): 731–738. December 2020. doi:10.1111/bju.15161. PMID 32633020.
- ↑ "Bipolar plasma vaporization vs monopolar and bipolar TURP-A prospective, randomized, long-term comparison". Urology 78 (4): 930–935. October 2011. doi:10.1016/j.urology.2011.03.072. PMID 21802121.
- ↑ "Comparison of Safety, Efficacy and Cost Effectiveness of Photoselective Vaporization with Bipolar Vaporization of Prostate in Benign Prostatic Hyperplasia" (in en-US). Current Urology 11 (2): 103–109. February 2018. doi:10.1159/000447202. PMID 29593470.
- ↑ "180-W XPS GreenLight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European Multicentre Randomised Trial--the GOLIATH study". European Urology 65 (5): 931–942. May 2014. doi:10.1016/j.eururo.2013.10.040. PMID 24331152.
- ↑ "Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate--a prospective, randomized, and controlled clinical trial". Radiology 270 (3): 920–928. March 2014. doi:10.1148/radiol.13122803. PMID 24475799.
- ↑ "Efficacy and Safety of Rezūm System Water Vapor Treatment for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia". Urology 86 (5): 1042–1047. November 2015. doi:10.1016/j.urology.2015.05.046. PMID 26216644.
- ↑ "PD18-04 Urolife and Rezum: A Comparison of Device Related Adverse Events in a National Registry" (in en). Journal of Urology 206 (Supplement 3). September 2021. doi:10.1097/JU.0000000000002007.04. ISSN 0022-5347. http://www.auajournals.org/doi/10.1097/JU.0000000000002007.04.
- ↑ "Randomised Clinical Trial of Prostatic Artery Embolisation Versus a Sham Procedure for Benign Prostatic Hyperplasia". European Urology 77 (3): 354–362. March 2020. doi:10.1016/j.eururo.2019.11.010. PMID 31831295.
- ↑ "Three-year outcomes after Aquablation therapy compared to TURP: results from a blinded randomized trial". The Canadian Journal of Urology 27 (1): 10072–10079. February 2020. 2020. PMID 32065861. https://www.canjurol.com/html/free-articles/Cdn_JU27_I1_05_FREE_DrGilling.pdf.
- ↑ "WATER II (80-150 mL) procedural outcomes". BJU International 123 (1): 106–112. January 2019. doi:10.1111/bju.14360. PMID 29694702.
- ↑ "WATER II Trial (Aquablation)" (in en). Current Bladder Dysfunction Reports 15 (3): 225–228. 2020-09-01. doi:10.1007/s11884-020-00596-y. ISSN 1931-7220. https://link.springer.com/article/10.1007/s11884-020-00596-y.
- ↑ "Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at 1 year of follow-up". BJU International 116 (2): 278–287. August 2015. doi:10.1111/bju.12982. PMID 25382816.
- ↑ "An Evaluation of Sexual Function in the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia in Men Treated with the Temporarily Implanted Nitinol Device". Journal of Endourology 37 (1): 74–79. January 2023. doi:10.1089/end.2022.0226. PMID 36070450.
- ↑ "Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study". World Journal of Urology 38 (12): 3235–3244. December 2020. doi:10.1007/s00345-020-03140-z. PMID 32124019.
- ↑ "Phytotherapy for Benign Prostatic Hyperplasia". Current Urology Reports 17 (7). July 2016. doi:10.1007/s11934-016-0609-z. PMID 27180172.
- ↑ "Saw palmetto for benign prostatic hyperplasia". The New England Journal of Medicine 354 (6): 557–566. February 2006. doi:10.1056/NEJMoa053085. PMID 16467543.
- ↑ "Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia". The Journal of Urology 179 (6): 2119–2125. June 2008. doi:10.1016/j.juro.2008.01.094. PMID 18423748.
- ↑ "Serenoa repens for the treatment of lower urinary tract symptoms due to benign prostatic enlargement". The Cochrane Database of Systematic Reviews 2023 (6). June 2023. doi:10.1002/14651858.CD001423.pub4. PMID 37345871.
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. https://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html.
- ↑ "Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care--the Triumph project". European Urology 42 (4): 323–328. October 2002. doi:10.1016/S0302-2838(02)00354-8. PMID 12361895.
External links
| Classification | |
|---|---|
| External resources |
 |
 KSF
KSF

