Capnography
Topic: Medicine
 From HandWiki - Reading time: 14 min
From HandWiki - Reading time: 14 min
| Capnography | |
|---|---|
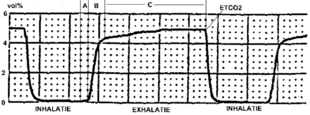 Typical capnogram. Normal breath cycle. | |
| Other names | End tidal CO2 (PETCO2) |
| MeSH | D019296 |
Capnography is the monitoring of the concentration or partial pressure of carbon dioxide (CO2) in the respiratory gases. Its main development has been as a monitoring tool for use during anesthesia and intensive care. It is usually presented as a graph of CO2 (measured in kilopascals, "kPa" or millimeters of mercury, "mmHg") plotted against time, or, less commonly, but more usefully, expired volume (known as volumetric capnography). The plot may also show the inspired CO2, which is of interest when rebreathing systems are being used. When the measurement is taken at the end of a breath (exhaling), it is called "end tidal" CO2 (PETCO2).[1]
The capnogram is a direct monitor of the inhaled and exhaled concentration or partial pressure of CO2, and an indirect monitor of the CO2 partial pressure in the arterial blood. In healthy individuals, the difference between arterial blood and expired gas CO2 partial pressures is very small (normal difference 4-5 mmHg). In the presence of most forms of lung disease, and some forms of congenital heart disease (the cyanotic lesions) the difference between arterial blood and expired gas increases which can be an indication of new pathology or change in the cardiovascular-ventilation system.[2] [3]
Medical Use
Oxygenation and capnography, although related, remain distinct elements in the physiology of respiration. Ventilation refers to the mechanical process of which the lungs expand and exchange volumes of gasses, however respiration further describes the exchange of gasses (mainly CO2 and O2) at the level of the alveoli. The process of respiration can be divided into two main functions: elimination of CO2 waste and replenishing tissues with fresh O2. Oxygenation (typically measured via pulse oximetry) measures the latter portion of this system. Capnography measures the elimination of CO2 which may be of greater clinical usefulness than oxygenation status.[4]
During the normal cycle of respiration, a single breath can be divided into two phases: inspiration and expiration. At the beginning of inspiration, the lungs expand and CO2 free gasses fill the lungs. As the alveoli are filled with this new gas, the concentration of CO2 that fills the alveoli is dependent on the ventilation of the alveoli and the perfusion (blood flow) that is delivering the CO2 for exchange. Once expiration begins to occur, the lung volume decreases as air is forced out the respiratory tract. The volume of CO2 that is exhaled at the end of exhalation is generated as a by product of metabolism from tissue throughout the body. The delivery of CO2 to the alveoli for exhalation is dependent on an intact cardiovascular system to ensure adequate blood flow from the tissue to the alveoli. If cardiac output (the amount of blood that is pumped out of the heart) is decreased, the ability to transport CO2 is also decreased which is reflected in a decreased expired amount of CO2. The relationship of cardiac output and end tidal CO2 is linear, such that as cardiac output increases or decreases, the amount of CO2 is also adjusted in the same manner. Therefore the monitoring of end tidal CO2 can provide vital information on the integrity of the cardiovascular system, specifically how well the heart is able to pump blood.[5]
The amount of CO2 that is measured during each breath requires an intact cardiovascular system to delivery the CO2 to the alveoli which is the functional unit of the lungs. During phase I of expiration, the CO2 transported to the lungs gas occupies a given space that is not involved in gas exchange, called dead space. Phase II of expiration is when the CO2 within the lungs is forced up the respiratory tract on its way to leave the body, which causes mixing of the air from the dead space with the air in the functional alveoli responsible for gas exchange. Phase III is the final portion of expiration which reflects CO2 only from the alveoli and not the dead space. These three phases are important to understand in clinical scenarios since a change in the shape and absolute values can indicate respiratory and/or cardiovascular compromise.[6]
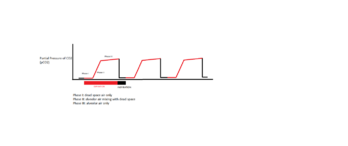
Applications
- Assessing Airway Integrity
- Confirmation of Endotracheal Tube Placement
- Predictor of Outcomes in the Intensive Care Unit
- Intraoperative Complications (ie. air embolism, thromboembolism, etc.)
- CPR use in ACLS (Advanced Cardiovascular Life Support)
- Procedural Sedation Monitoring
Anesthesia

During anesthesia, there is interplay between two components: the patient and the anesthesia administration device (which is usually a breathing circuit and a ventilator). The critical connection between the two components is either an endotracheal tube or a mask, and CO2 is typically monitored at this junction. Capnography directly reflects the elimination of CO2 by the lungs to the anesthesia device. Indirectly, it reflects the production of CO2 by tissues and the circulatory transport of CO2 to the lungs.[7]
When expired CO2 is related to expired volume rather than time, the area beneath the curve represents the volume of CO2 in the breath, and thus over the course of a minute, this method can yield the CO2 per minute elimination, an important measure of metabolism. Sudden changes in CO2 elimination during lung or heart surgery usually imply important changes in cardiorespiratory function.[8]
Capnography has been shown to be more effective than clinical judgement alone in the early detection of adverse respiratory events such as hypoventilation, esophageal intubation and circuit disconnection; thus allowing patient injury to be prevented. During procedures done under sedation, capnography provides more useful information, e.g. on the frequency and regularity of ventilation, than pulse oximetry.[9][10]
Capnography provides a rapid and reliable method to detect life-threatening conditions (malposition of tracheal tubes, unsuspected ventilatory failure, circulatory failure and defective breathing circuits) and to circumvent potentially irreversible patient injury.
Capnography and pulse oximetry together could have helped in the prevention of 93% of avoidable anesthesia mishaps according to an ASA (American Society of Anesthesiologists) closed claim study.[11]
Emergency medical services
Capnography is increasingly being used by EMS personnel to aid in their assessment and treatment of patients in the prehospital environment. These uses include verifying and monitoring the position of an endotracheal tube or a blind insertion airway device. A properly positioned tube in the trachea guards the patient's airway and enables the paramedic to breathe for the patient. A misplaced tube in the esophagus can lead to the patient's death if it goes undetected. [12]
A study in the March 2005 Annals of Emergency Medicine, comparing field intubations that used continuous capnography to confirm intubations versus non-use showed zero unrecognized misplaced intubations in the monitoring group versus 23% misplaced tubes in the unmonitored group.[13] The American Heart Association (AHA) affirmed the importance of using capnography to verify tube placement in their 2005 CPR and Emergency Cardiovascular Care Guidelines.[14]
The AHA also notes in their new guidelines that capnography, which indirectly measures cardiac output, can also be used to monitor the effectiveness of CPR and as an early indication of return of spontaneous circulation (ROSC). Studies have shown that when a person doing CPR tires, the patient's end-tidal CO2 (PETCO2, the level of carbon dioxide released at the end of expiration) falls, and then rises when a fresh rescuer takes over. Other studies have shown when a patient experiences return of spontaneous circulation, the first indication is often a sudden rise in the PETCO2 as the rush of circulation washes untransported CO2 from the tissues. Likewise, a sudden drop in PETCO2 may indicate the patient has lost pulses and CPR may need to be initiated.[15]
Paramedics are also now beginning to monitor the PETCO2 status of nonintubated patients by using a special nasal cannula that collects the carbon dioxide. A high PETCO2 reading in a patient with altered mental status or severe difficulty breathing may indicate hypoventilation and a possible need for the patient to be intubated. Low PETCO2 readings on patients may indicate hyperventilation.[16]
Capnography, because it provides a breath by breath measurement of a patient's ventilation, can quickly reveal a worsening trend in a patient's condition by providing paramedics with an early warning system into a patient's respiratory status. When compared to oxygenation which is measured by pulse oximetry, there are several disadvantages that capnography can help address to provide a more accurate reflection of cardiovascular integrity. One shortcoming of measuring pulse oximetry alone is that administration of supplemental oxygen (ie. via nasal cannula) can delay desaturation in a patient if they stopped breathing, therefore delaying medical intervention. Capnography provides a rapid way to directly assess ventilation status and indirectly assess cardiac function. Clinical studies are expected to uncover further uses of capnography in asthma, congestive heart failure, diabetes, circulatory shock, pulmonary embolus, acidosis, and other conditions, with potential implications for the prehospital use of capnography.[17]
Registered nurses
Registered nurses, but more so RRTs (respiratory therapists), in critical care settings may use capnography to determine if a nasogastric tube, which is used for feeding, has been placed in the trachea as opposed to the esophagus.[18] Usually a patient will cough or gag if the tube is misplaced, but most patients in critical care settings are sedated or comatose. If a nasogastric tube is accidentally placed in the trachea instead of the esophagus, the tube feedings will go into the lungs, which is a life-threatening situation. If the monitor displays typical CO2 waveforms then placement should be confirmed.[19]
Diagnostic usage
Capnography provides information about CO2 production, pulmonary (lung) perfusion, alveolar ventilation, respiratory patterns, and elimination of CO2 from the anesthesia breathing circuit and ventilator. The shape of the curve is affected by some forms of lung disease; in general there are obstructive conditions such as bronchitis, emphysema and asthma, in which the mixing of gases within the lung is affected.[20]
Conditions such as pulmonary embolism and congenital heart disease, which affect perfusion of the lung, do not, in themselves, affect the shape of the curve, but greatly affect the relationship between expired CO2 and arterial blood CO2. Capnography can also be used to measure carbon dioxide production, a measure of metabolism. Increased CO2 production is seen during fever and shivering. Reduced production is seen during anesthesia and hypothermia.[21]
Working mechanism
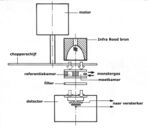
Capnographs work on the principle that CO2 is a polyatomic gas and therefore absorbs infrared radiation. A beam of infrared light is passed across the gas sample to fall on a sensor. The presence of CO2 in the gas leads to a reduction in the amount of light falling on the sensor, which changes the voltage in a circuit. The analysis is rapid and accurate, but the presence of nitrous oxide in the gas mix changes the infrared absorption via the phenomenon of collision broadening.[22] This must be corrected for measuring the CO2 in human breath by measuring its infrared absorptive power. This was established as a reliable technique by John Tyndall in 1864, though 19th and early 20th century devices were too cumbersome for everyday clinical use.[23] Today, technologies have since improved and are able to measure the values of CO2 near instantaneously and has become a standard practice in medical settings. There are currently two main types of CO2 sensors that are used in clinical practice: main-stream sensors and side-stream sensors. Both effectively serve the same function to quantify the amount of CO2 that is being exhaled in each breath.
Capnogram model
The capnogram waveform provides information about various respiratory and cardiac parameters. The capnogram double-exponential model attempts to quantitatively explain the relationship between respiratory parameters and the exhalatory segment of a capnogram waveform.[24] According to the model, each exhalatory segment of capnogram waveform follows the analytical expression:
where
- represents the partial pressure of carbon dioxide measured by the capnogram as a function of time since the beginning of exhalation.
- represents the alveolar partial pressure of carbon dioxide.
- represents the inverse of the dead space fraction (i.e. the ratio of tidal volume to dead space volume).
- represents the pulmonary time constant (i.e. the product of pulmonary resistance and compliance)
In particular, this model explains the rounded "shark-fin" shape of the capnogram observed in patients with obstructive lung disease.
See also
- Integrated pulmonary index
- Medical equipment
- Medical test
- Respiratory monitoring
- Colorimetric capnography
Citations
- ↑ Bhavani-Shankar, Kodali; Philip, James (October 2000). "Defining segments and phases of a time capnogram" (in en). Anesth Analg 91 (4): 973–977. doi:10.1097/00000539-200010000-00038. PMID 11004059.
- ↑ Nunn, J; Hill, D (May 1960). "Respiratory dead space and arterial to end-tidal carbon dioxide tension difference in anesthetized man" (in en). J Appl Physiol 15: 383–389. doi:10.1152/jappl.1960.15.3.383. PMID 14427915.
- ↑ Williams, Emma; Dassios, Theodore; Greenough, Anne (October 2021). "Carbon dioxide monitoring in the newborn" (in en). Pediatr Pulmonol 56 (10): 3148–3156. doi:10.1002/ppul.25605. PMID 34365738.
- ↑ Lam, Thach; Nagappa, Mahesh; Wong, Jean; Singh, Mandeep; Wong, David; Chung, Frances (December 2017). "Continuous Pulse Oximetry and Capnography Monitoring for Postoperative Respiratory Depression and Adverse Events: A Systematic Review and Meta-analysis" (in en). Anesthesia & Analgesia 125 (6): 2019–2029. doi:10.1213/ANE.0000000000002557. ISSN 0003-2999. PMID 29064874. http://journals.lww.com/00000539-201712000-00031.
- ↑ Siobal, Mark (October 2016). "Monitoring Exhaled Carbon Dioxide" (in en). Respir Care 61 (10): 1397–1416. doi:10.4187/respcare.04919. PMID 27601718.
- ↑ Benumof, Jeffrey (April 1998). "Interpretation of capnography" (in en). AANA J 661 (2): 169–176.
- ↑ Weil, Max; Bisera, Jose; Trevino; Rackow, Eric (October 2016). "Cardiac output and end-tidal carbon dioxide" (in en). Crit Care Med 13 (11): 907–909. doi:10.1097/00003246-198511000-00011. PMID 3931979.
- ↑ Capnography (2 ed.). Cambridge University Press. 17 March 2011. ISBN 978-0-521-51478-1. OCLC 1031490358. https://books.google.com/books?id=9hb6mAEACAAJ.
- ↑ Lightdale, Jenifer R.; Goldmann, Donald A.; Feldman, Henry A.; Newburg, Adrienne R.; DiNardo, James A.; Fox, Victor L. (June 2006). "Microstream capnography improves patient monitoring during moderate sedation: a randomized, controlled trial". Pediatrics 117 (6): e1170–1178. doi:10.1542/peds.2005-1709. ISSN 1098-4275. PMID 16702250. https://pubmed.ncbi.nlm.nih.gov/16702250/.
- ↑ Burton, John H.; Harrah, John D.; Germann, Carl A.; Dillon, Douglas C. (May 2006). "Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices?". Academic Emergency Medicine 13 (5): 500–504. doi:10.1197/j.aem.2005.12.017. ISSN 1553-2712. PMID 16569750. https://pubmed.ncbi.nlm.nih.gov/16569750/.
- ↑ Tinker, John H.; Dull, David L.; Caplan, Robert A.; Ward, Richard J.; Cheney, Frederick W. (1989). "Role of Monitoring Devices in Prevention of Anesthetic Mishaps". Anesthesiology 71 (4): 541–546. doi:10.1097/00000542-198910000-00010. PMID 2508510.
- ↑ Katz, Steven; Falk, Jay (January 2001). "Misplaced endotracheal tubes by paramedics in an urban emergency medical services system" (in en). Ann Emerg Med 37 (1): 32–37. doi:10.1067/mem.2001.112098. PMID 11145768. https://www.annemergmed.com/article/S0196-0644(01)92235-8/fulltext.
- ↑ Silvestri, Salvatore; Ralls, George A.; Krauss, Baruch; Thundiyil, Josef; Rothrock, Steven G.; Senn, Amy; Carter, Eric; Falk, Jay (May 2005). "The effectiveness of out-of-hospital use of continuous end-tidal carbon dioxide monitoring on the rate of unrecognized misplaced intubation within a regional emergency medical services system". Annals of Emergency Medicine 45 (5): 497–503. doi:10.1016/j.annemergmed.2004.09.014. ISSN 1097-6760. PMID 15855946. https://pubmed.ncbi.nlm.nih.gov/15855946/.
- ↑ Hazinski, Mary Fran; Nadkarni, Vinay M.; Hickey, Robert W.; O'Connor, Robert; Becker, Lance B.; Zaritsky, Arno (2005-12-13). "Major Changes in the 2005 AHA Guidelines for CPR and ECC". Circulation 112 (24_supplement): IV–206. doi:10.1161/CIRCULATIONAHA.105.170809. PMID 16314349.
- ↑ Long, Brit; Koyfman, Alex; Vivirito, Michael A. (December 2017). "Capnography in the Emergency Department: A Review of Uses, Waveforms, and Limitations". The Journal of Emergency Medicine 53 (6): 829–842. doi:10.1016/j.jemermed.2017.08.026. ISSN 0736-4679. PMID 28993038.
- ↑ Davis, Daniel; Dunford, James; Ochs, Mel; Park, Kenneth; Hoyt, David (April 2004). "The use of quantitative end-tidal capnometry to avoid inadvertent severe hyperventilation in patients with head injury after paramedic rapid sequence intubation" (in en). J Trauma 56 (4): 808–814. doi:10.1097/01.TA.0000100217.05066.87. PMID 15187747.
- ↑ "Experts: Where capnography is headed" (in en). 20 November 2013. https://www.ems1.com/ems-products/medical-equipment/airway-management/articles/experts-where-capnography-is-headed-bXd5NUiEucMcXOOg/.
- ↑ Potter, Patricia Ann, and Anne Griffin Perry. "Nutrition." Essentials for nursing practice. Eighth ed. St. Louis: Elsevier, 2015. 940. Print.
- ↑ Roubenoff, Ronenn; Ravich, William (April 1998). "Pneumothorax due to nasogastric feeding tubes. Report of four cases, review of the literature, and recommendations for prevention" (in en). Arch Intern Med 149 (1): 184–188. doi:10.1001/archinte.1989.00390010156022. PMID 2492185.
- ↑ Yaron, Michael; Padyk, Paul; Hutsinpiller, Molly; Cairns, Charles (October 1996). "Utility of the expiratory capnogram in the assessment of bronchospasm" (in en). Ann Emerg Med 28 (4): 403–407. doi:10.1016/S0196-0644(96)70005-7. PMID 8839525.
- ↑ Danzl, Daniel (February 2002). "Hypothermia system" (in en). Semin Respir Crit Care Med 23 (1): 57–68. doi:10.1055/s-2002-20589. PMID 16088598.
- ↑ "Accuracy of end-tidal carbon dioxide tension analyzers". J Clin Monit 7 (2): 195–208. April 1991. doi:10.1007/BF01618124. PMID 1906531.
- ↑ Jaffe MB (September 2008). "Infrared measurement of carbon dioxide in the human breath: "breathe-through" devices from Tyndall to the present day". Anesth. Analg. 107 (3): 890–904. doi:10.1213/ane.0b013e31817ee3b3. PMID 18713902.
- ↑ Abid, Abubakar (May 2017). "Model-Based Estimation of Respiratory Parameters from Capnography, With Application to Diagnosing Obstructive Lung Disease". IEEE Transactions on Biomedical Engineering 64 (12): 2957–2967. doi:10.1109/TBME.2017.2699972. PMID 28475040.
External links
- CapnoBase.org: Respiratory signal database that contains clinical and simulated capnogram recordings
 |
 KSF
KSF