Fontan procedure
Topic: Medicine
 From HandWiki - Reading time: 9 min
From HandWiki - Reading time: 9 min
| Fontan procedure | |
|---|---|
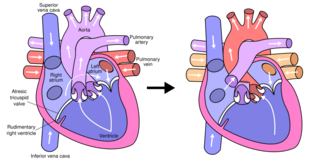 Fontan procedure for tricuspid atresia | |
| Uses | Palliative surgery for child with univentricular heart |
The Fontan procedure or Fontan–Kreutzer procedure is a palliative surgical procedure used in children with univentricular hearts. It involves diverting the venous blood from the inferior vena cava (IVC) and superior vena cava (SVC) to the pulmonary arteries. The procedure varies for differing congenital heart pathologies. For example in tricuspid atresia, the procedure can be done where the blood does not pass through the morphologic right ventricle; i.e., the systemic and pulmonary circulations are placed in series with the functional single ventricle. Whereas in hypoplastic left heart syndrome, the heart is more reliant on the more functional right ventricle to provide blood flow to the systemic circulation. The procedure was initially performed in 1968 by Francis Fontan and Eugene Baudet from Bordeaux, France, published in 1971, simultaneously described in 1971 by Guillermo Kreutzer from Buenos Aires, Argentina, and finally published in 1973.[1][2]
Indications
The Fontan Kreutzer procedure is used in pediatric patients who possess only a single functional ventricle, either due to lack of a heart valve (e.g. tricuspid or mitral atresia), an abnormality of the pumping ability of the heart (e.g. hypoplastic left heart syndrome or hypoplastic right heart syndrome), or a complex congenital heart disease where a bi-ventricular repair is impossible or inadvisable. The surgery allows blood to be delivered to the lungs via central venous pressure rather than via the right ventricle.[3] Patients typically present as neonates with cyanosis or congestive heart failure.[4] Fontan completion is usually carried out when the patient is 2–5 years of age, but is also performed before 2 years of age.[5][6]
Types
There are four variations of the Fontan procedure:[7]
- Ventricularization of the Right Atrium (The original Fontan's Technique)
- Atriopulmonary connection (the original Kreutzer's Technique)
- Intracardiac total cavopulmonary connection (lateral tunnel) (described by Marc De Leval and Aldo Castañeda, separately)
- Extracardiac total cavopulmonary connection (described by Carlo Marceletti and Francisco Puga for Heterotaxy Syndrome)
Approach

The Fontan procedure is the third procedure in the staged surgical palliation.[8] It is performed in children born with congenital heart disease without two functional ventricles and an effective parallel blood flow circuit.[9]
The first stage is known as the Norwood procedure. This stage generally involves combining the pulmonary artery and aorta to form a larger vessel for blood to get to the body. An artificial tube or shunt can be placed from this larger vessel to the pulmonary arteries so that blood can get from the heart to the lungs. The wall between the left and right atrium can be removed to allow the mixing of oxygenated and de-oxygenated blood.[10][11][12]
The second stage is called the hemi-Fontan or the Bidirectional Glenn procedure. This intermediary stage incorporates the shifting of oxygen-poor blood from the top of the body to the lungs.[13] The superior vena cava (SVC), which carries blood returning from the upper parts of the body, is disconnected from the heart and instead redirects the blood into the pulmonary arteries.[13] The inferior vena cava (IVC), which carries blood returning from the lower body, continues to connect to the right atrium.[14][12]
The third stage is called the Fontan procedure which involves redirecting the blood from the inferior vena cava to the lungs.[8] At this point, the oxygen-poor blood from upper and lower body flows through the lungs without being pumped (driven only by the pressure that builds up in the veins or central venous pressure). This improves the lower than normal oxygen levels and results in one functional ventricle that is responsible for supplying blood to the rest of the body. There are currently three various modern techniques for the Fontan procedure which include: Atriopulmonary connection, lateral tunnel total cavopulmonary connection, and extracardiac conduit.
Contraindications
After Fontan Kreutzer completion, blood must flow through the lungs without being pumped by the heart. Therefore, children with high pulmonary vascular resistance may not tolerate a Fontan procedure. Often, cardiac catheterization is performed to check the resistance before proceeding with the surgery. This is also the reason a Fontan procedure cannot be done immediately after birth; the pulmonary vascular resistance is high in utero and takes months to drop. Fontan procedure is also contraindicated in those with pulmonary artery hypoplasia and significant mitral insufficiency.[citation needed]
Post-operative complications
In the short term, children can have trouble with pleural effusions (fluid building up around the lungs). This can require a longer stay in the hospital for drainage with chest tubes. To address this risk, some surgeons make a fenestration from the venous circulation into the atrium. When the pressure in the veins is high, some of the oxygen-poor blood can escape through the fenestration to relieve the pressure. However, this results in hypoxia, so the fenestration may eventually need to be closed by an interventional cardiologist.
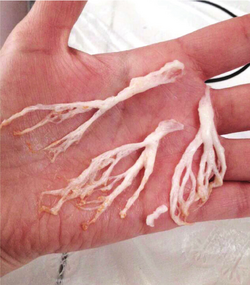
In a 2016 review, Dr. Jack Rychik, head of the Single Ventricle Survivorship Program at Children's Hospital of Philadelphia summarized the long-term consequences of Fontan circulation as an "indolent and progressive state of heart failure" with predictable long-term consequences on several organ systems.[15] Chronic venous hypertension from the stasis and lowered cardiac output are assumed to be at the root of lymphatic complications such as chylothorax, protein losing enteropathy and plastic bronchitis. These complications may occur in the immediate post-operative period as well as in the medium and long term. New interventional and surgical strategies have been investigated to relieve the lymphatic complications associated with the Fontan circulation.[16] Concerns about damage to the liver have emerged more recently, as the Fontan circulation produces congestion and lymphedema in this organ. This can lead towards progressive hepatic fibrosis and other complications of Fontan-Associated Liver Disease.[17] Screening protocols and treatment standards are emerging in the light of these discoveries.[15]
Because of structural and electrochemical changes related to scarring after the procedure, arrhythmias are common. Pacemakers are placed in as many as 7% of patients who undergo the Fontan procedure.[18] While the need for pacemakers may be related to the underlying cardiac anomaly, there is sufficient evidence that the surgery itself lead to the need for cardiac pacing.
The Fontan procedure is palliative — not curative — but more than 80% of the cases can result in normal or near-normal growth, development, exercise tolerance, and good quality of life.[19] However, 10% or more of patients may eventually require heart transplantation,[20] and given the long-term consequences of chronic venous hypertension and insidious organ damage, freedom from morbidity is unlikely in the long term. New approaches to the management of failing Fontans or other clinical deterioration have included lymphatic decompression surgical procedures & intervention, Ventricular assist devices or other mechanical support therapies as either bridge to transplantation or destination therapies.[21]
Renal complications may occur. This is attributed to the circulatory changes in blood flow as well as possible exposure to nephrotoxic medications, iodine contrast agents, and long term cyanotic and ischemic nephropathy. Abnormalities including chronic kidney disease and impaired renal function have been shown with measured renal function.[22] Popular markers, such as proteinuria and microalbuminuria, are used in the measurement renal function.
It is estimated in 2018 there was an 85% for a survival rate of thirty years following a Fontan procedure and there are approximately 50,000 to 70,000 people in the world with Fontan circulation.[23] It is approximated that 40% of people with Fontan circulation are ≥18 years of age.[22]
Fontan circulation
A normal heart system has a series circuit with the right ventricle pumping blood into the pulmonary circulation which, after exchanging gases, delivers it to the left ventricle (and systemic arteries) through pulmonary veins. Because of this series arrangement blood flow (cardiac output) is the same in pulmonary and systemic circulations, as electrical current is the same across series resistances. In a Fontan Circulation, the right ventricle does not exist (or is bypassed) and the venae cavae are attached directly to the pulmonary artery.[24] After oxygenation, the blood is pumped in systemic arteries (aorta) by the unique ventricle. Because of the missing right ventricle, the force driving blood through the lung is strongly reduced, thus causing engorgement of the venous circulation, the most frequent complication of the Fontan procedure.
Pregnancy considerations
The Fontan circulation can influence the peripartum or pregnant physiologic states. Pregnancy has historically been discouraged due to high rates of miscarriage, cardiovascular compromise, or increased mortality. Many complications have been attributed to flawed placental function. This may be attributed to However, improvements of the Fontan operation have resulted in pregnancies with lower incidence of heart and vascular compromise in the mother.[25] Complications that may occur in the fetus may include but is not limited to oligohydramnios, preterm birth, low birth weight, small gestational age, or still birth. Maternal complications can include but is not limited to heart failure, thromboembolism, arrhythmias, and preeclampsia.[22]
History
The Fontan procedure was initially described in 1971 by Dr. Francis Fontan (1929–2018) from Bordeaux, France. Prior to this, the surgical treatment for tricuspid atresia consisted of creating a shunt between a systemic artery and the pulmonary artery (Blalock-Taussig shunt) or the superior vena cava and the pulmonary artery (Glenn shunt). These procedures were associated with high mortality rates, commonly leading to death before the age of one year.[26] In an attempt to improve this, Fontan was engaged in research between 1964 and 1966 endeavouring to fully redirect flow from the superior and inferior vena cavae to the pulmonary artery.[27] His initial attempts in dogs were unsuccessful and all experimental animals died within a few hours; however, despite these failures, he successfully performed this operation in a young woman with tricuspid atresia in 1968 with Dr Eugene Baudet.[26] The operation was completed on a second patient in 1970, and after a third case the series was published in the international journal Thorax in 1971.[28] Dr. Guillermo Kreutzer from Buenos Aires, Argentina (b. 1934) without any knowledge of Fontan's experience performed a similar procedure in July, 1971 without placing a valve in the Inferior Vena Cava inlet and introducing the concept of "fenestration" leaving a small atrial septal defect to serve as a pop-off valve for the circulation.[29][26] Techniques have been improved to include the lateral tunnel and use of an extracardiac conduit for congenital heart diseases beyond tricuspid atresia (hypoplastic left heart syndrome, etc).[30]
References
- ↑ Fontan, F.; Baudet, E. (May 1971). "Surgical repair of tricuspid atresia". Thorax 26 (3): 240–248. doi:10.1136/thx.26.3.240. ISSN 0040-6376. PMID 5089489.
- ↑ Kreutzer, G.; Galíndez, E.; Bono, H.; De Palma, C.; Laura, J. P. (October 1973). "An operation for the correction of tricuspid atresia". The Journal of Thoracic and Cardiovascular Surgery 66 (4): 613–621. doi:10.1016/S0022-5223(19)40598-9. ISSN 0022-5223. PMID 4518787. https://pubmed.ncbi.nlm.nih.gov/4518787/.
- ↑ "Single Ventricle". StatPearls. Treasure Island (FL): StatPearls Publishing. 2021. http://www.ncbi.nlm.nih.gov/books/NBK557789/. Retrieved 2021-08-18.
- ↑ "Prevalence, clinical presentation and natural history of patients with single ventricle.". Progress in Pediatric Cardiology 16: 31–38. 2002. doi:10.1016/s1058-9813(02)00042-5.
- ↑ "Fontan operation in the current era: a 15-year single institution experience". Annals of Surgery 248 (3): 402–10. September 2008. doi:10.1097/SLA.0b013e3181858286. PMID 18791360.
- ↑ "Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study". Journal of the American College of Cardiology 52 (2): 85–98. July 2008. doi:10.1016/j.jacc.2008.01.074. PMID 18598886.
- ↑ "The Fontan circulation: a challenge to William Harvey?". Nature Clinical Practice. Cardiovascular Medicine 2 (4): 202–8. April 2005. doi:10.1038/ncpcardio0157. PMID 16265484.
- ↑ 8.0 8.1 Ohye, Richard G.; Schranz, Dietmar; D’Udekem, Yves (2016-10-25). "Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions". Circulation 134 (17): 1265–1279. doi:10.1161/circulationaha.116.022816. ISSN 0009-7322. PMID 27777296. PMC 5119545. http://dx.doi.org/10.1161/circulationaha.116.022816.
- ↑ Rocha Martinez, Tania Leme da (2022-04-30). "Successful Palliation in Monochorionic Twins with Hypoplastic Left Heart Syndrome". Cardiology Research and Reports 4 (4): 01–02. doi:10.31579/2692-9759/047. ISSN 2692-9759.
- ↑ Schranz, Dietmar; Esmaeili, Anoosh; Akintuerk, Hakan (April 2021). "Hypoplastic Left Heart: Stage-I Will be Performed Interventionally, Soon". Pediatric Cardiology 42 (4): 727–735. doi:10.1007/s00246-021-02597-y. ISSN 0172-0643. PMID 33871681. PMC 8110497. http://dx.doi.org/10.1007/s00246-021-02597-y.
- ↑ Yabrodi, Mouhammad; Mastropietro, Christopher W. (2016-10-04). "Hypoplastic left heart syndrome: from comfort care to long-term survival". Pediatric Research 81 (1–2): 142–149. doi:10.1038/pr.2016.194. ISSN 0031-3998. PMID 27701379. PMC 5313512. http://dx.doi.org/10.1038/pr.2016.194.
- ↑ 12.0 12.1 Donnelly, Jon P.; Raffel, David M.; Shulkin, Barry L.; Corbett, James R.; Bove, Edward L.; Mosca, Ralph S.; Kulik, Thomas J. (January 1998). "Resting coronary flow and coronary flow reserve in human infants after repair or palliation of congenital heart defects as measured by positron emission tomography". The Journal of Thoracic and Cardiovascular Surgery 115 (1): 103–110. doi:10.1016/s0022-5223(98)70448-9. ISSN 0022-5223. PMID 9451052.
- ↑ 13.0 13.1 Yuan, Shi-Min; Jing, Hua (June 2009). "Palliative procedures for congenital heart defects". Archives of Cardiovascular Diseases 102 (6–7): 549–557. doi:10.1016/j.acvd.2009.04.011. ISSN 1875-2136. PMID 19664575.
- ↑ van der Ven, Jelle P. G.; van den Bosch, Eva; Bogers, Ad J.C.C.; Helbing, Willem A. (2018-06-27). "State of the art of the Fontan strategy for treatment of univentricular heart disease". F1000Research 7: F1000 Faculty Rev-935. doi:10.12688/f1000research.13792.1. ISSN 2046-1402. PMID 30002816.
- ↑ 15.0 15.1 "The Relentless Effects of the Fontan Paradox". Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual 19 (1): 37–43. 2016. doi:10.1053/j.pcsu.2015.11.006. PMID 27060041.
- ↑ Hraska V, Hjortdal VE, Dori Y, Kreutzer C | title = Innominate vein turn-down procedure: Killing two birds with one stone | journal = Journal of Thoracic and cardiovascular Surgery Tech. 2021;7: | volume = 7 | issue = 1 | pages = 253-260 | date = Jun 2021 | pmid = 34318266 | doi =10.1016/j.xjtc.2021.01.045
- ↑ "Fontan-associated liver disease: A review". Journal of Cardiology 74 (3): 223–232. September 2019. doi:10.1016/j.jjcc.2019.02.016. PMID 30928109.
- ↑ Cohen, M. I.; Wernovsky, G.; Vetter, V. L.; Wieand, T. S.; Gaynor, J. W.; Jacobs, M. L.; Spray, T. L.; Rhodes, L. A. (1998-11-10). "Sinus node function after a systematically staged Fontan procedure". Circulation 98 (19 Suppl): II352–358; discussion II358–359. ISSN 0009-7322. PMID 9852926. https://pubmed.ncbi.nlm.nih.gov/9852926.
- ↑ "Surgical outcomes in complex adult congenital heart disease: a brief review". Journal of Thoracic Disease 12 (3): 1224–1234. March 2020. doi:10.21037/jtd.2019.12.136. PMID 32274204.
- ↑ "Transplantation of the failing Fontan". Translational Pediatrics 8 (4): 290–301. October 2019. doi:10.21037/tp.2019.06.03. PMID 31728322.
- ↑ "Managing adult Fontan patients: where do we stand?". European Respiratory Review 25 (142): 438–450. December 2016. doi:10.1183/16000617.0091-2016. PMID 27903666.
- ↑ 22.0 22.1 22.2 Rychik, Jack; Atz, Andrew M.; Celermajer, David S.; Deal, Barbara J.; Gatzoulis, Michael A.; Gewillig, Marc H.; Hsia, Tain-Yen; Hsu, Daphne T. et al. (2019-08-06). "Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association" (in en). Circulation 140 (6): e234–e284. doi:10.1161/CIR.0000000000000696. ISSN 0009-7322. PMID 31256636.
- ↑ Schilling, Chris; Dalziel, Kim; Nunn, Russell; Du Plessis, Karin; Shi, William Y.; Celermajer, David; Winlaw, David; Weintraub, Robert G. et al. (September 2016). "The Fontan epidemic: Population projections from the Australia and New Zealand Fontan Registry" (in en). International Journal of Cardiology 219: 14–19. doi:10.1016/j.ijcard.2016.05.035. PMID 27257850. https://linkinghub.elsevier.com/retrieve/pii/S0167527316309391.
- ↑ Gewillig, Marc; Brown, Stephen C (2016-07-15). "The Fontan circulation after 45 years: update in physiology" (in en). Heart 102 (14): 1081–1086. doi:10.1136/heartjnl-2015-307467. ISSN 1355-6037. PMID 27220691.
- ↑ Garcia Ropero, Alvaro; Baskar, Shankar; Roos Hesselink, Jolien W.; Girnius, Andrea; Zentner, Dominica; Swan, Lorna; Ladouceur, Magalie; Brown, Nicole et al. (May 2018). "Pregnancy in Women With a Fontan Circulation: A Systematic Review of the Literature". Circulation: Cardiovascular Quality and Outcomes 11 (5): e004575. doi:10.1161/CIRCOUTCOMES.117.004575. ISSN 1941-7705. PMID 29752389.
- ↑ 26.0 26.1 26.2 "The Fontan procedure: a historical review". The Annals of Thoracic Surgery 51 (6): 1026–30. June 1991. doi:10.1016/0003-4975(91)91044-v. PMID 2039305.
- ↑ "Francis Fontan". Cardiology in the Young 9 (6): 592–600. November 1999. doi:10.1017/s1047951100005631. PMID 10593269.
- ↑ "Surgical repair of tricuspid atresia". Thorax 26 (3): 240–8. May 1971. doi:10.1136/thx.26.3.240. PMID 5089489.
- ↑ "An operation for the correction of tricuspid atresia". The Journal of Thoracic and Cardiovascular Surgery 66 (4): 613–21. October 1973. doi:10.1016/S0022-5223(19)40598-9. PMID 4518787.
- ↑ Walker, Sally M. (2005). Secrets of a Civil War submarine : solving the mysteries of the H.L. Hunley. Minneapolis: Carolrhoda Books. ISBN 1-57505-830-8. OCLC 56368642. https://www.worldcat.org/oclc/56368642.
External links
 |
 KSF
KSF