Hormone replacement therapy
Topic: Medicine
 From HandWiki - Reading time: 40 min
From HandWiki - Reading time: 40 min
Hormone replacement therapy (HRT), also known as menopausal hormone therapy or postmenopausal hormone therapy, is a form of hormone therapy used to treat symptoms associated with female menopause.[1][2] These symptoms can include hot flashes, vaginal atrophy, accelerated skin aging, vaginal dryness, decreased muscle mass, sexual dysfunction, and bone loss or osteoporosis. They are in large part related to the diminished levels of sex hormones that occur during menopause.[1][2]
Estrogens and progestogens are the main hormone drugs used in HRT. Progesterone is the main female sex hormone that occurs naturally and is also manufactured into a drug that is used in menopausal hormone therapy.[1] Although both classes of hormones can have symptomatic benefit, progestogen is specifically added to estrogen regimens, unless the uterus has been removed, to avoid the increased risk of endometrial cancer. Unopposed estrogen therapy promotes endometrial hyperplasia and increases the risk of cancer, while progestogen reduces this risk.[3][4] Androgens like testosterone are sometimes used as well.[5] HRT is available through a variety of different routes.[1][2]
The long-term effects of HRT on most organ systems vary by age and time since the last physiological exposure to hormones, and there can be large differences in individual regimens, factors which have made analyzing effects difficult.[6] The Women's Health Initiative (WHI) is an ongoing study of over 27,000 women that began in 1991, with the most recent analyses suggesting that, when initiated within 10 years of menopause, HRT reduces all-cause mortality and risks of coronary disease, osteoporosis, and dementia; after 10 years the beneficial effects on mortality and coronary heart disease are no longer apparent, though there are decreased risks of hip and vertebral fractures and an increased risk of venous thromboembolism when taken orally.[7][8]
"Bioidentical" hormone replacement is a development in the 21st century and uses manufactured compounds with "exactly the same chemical and molecular structure as hormones that are produced in the human body."[9] These are mainly steroids derived from plants[10] and can be a component of either registered pharmaceutical or custom-made compounded preparations, with the latter generally not recommended by regulatory bodies due to their lack of standardization and formal oversight.[11] Bioidentical hormone replacement has inadequate clinical research to determine its safety and efficacy as of 2017.[12]
The current indications for use from the United States Food and Drug Administration (FDA) include short-term treatment of menopausal symptoms, such as vasomotor hot flashes or vaginal atrophy, and prevention of osteoporosis.[13]
Medical uses
Approved uses of HRT in the United States include short-term treatment of menopausal symptoms such as hot flashes and vaginal atrophy, and prevention of osteoporosis.[13] The American College of Obstetrics and Gynecology (ACOG) approves of HRT for symptomatic relief of menopausal symptoms,[14] and advocates its use beyond the age of 65 in appropriate scenarios.[15] The North American Menopause Society (NAMS) 2016 annual meeting mentioned that HRT may have more benefits than risks in women before the age of 60.[16]
A consensus expert opinion published by The Endocrine Society stated that when taken during perimenopause or the initial years of menopause, HRT carries fewer risks than previously published, and reduces all cause mortality in most scenarios.[17] The American Association of Clinical Endocrinologists (AACE) has also released position statements approving of HRT when appropriate.[12]
Women receiving this treatment are usually post-, peri-, or surgically induced menopausal. Menopause is the permanent cessation of menstruation resulting from loss of ovarian follicular activity, defined as beginning twelve months after the final natural menstrual cycle. This twelve month time point divides menopause into early and late transition periods known as 'perimenopause' and 'postmenopause'.[4] Premature menopause can occur if the ovaries are surgically removed, as can be done to treat ovarian or uterine cancer.
Demographically, the vast majority of data available is in postmenopausal American women with concurrent pre-existing conditions and an average age of over 60 years.[18]
Menopausal symptoms
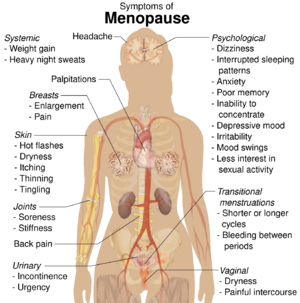
HRT is often given as a short-term relief from menopausal symptoms during perimenopause.[19] Potential menopausal symptoms include:[1][2]
- Hot flashes – vasomotor symptoms
- Vulvovaginal atrophy – atrophic vaginitis and dryness
- Dyspareunia – painful sexual intercourse due to vaginal atrophy and lack of lubrication
- Bone loss – decreased bone mineral density, which can eventually lead to osteopenia, osteoporosis, and associated fractures
- Decreased sexual desire
- Defeminization – diminished feminine fat distribution and accelerated skin aging[20][21]
- Sleep disturbances and joint pain
The most common of these are loss of sexual drive and vaginal dryness.[4][22]
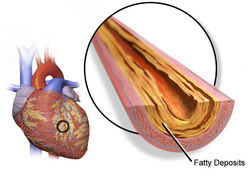
Heart disease
The effect of HRT in menopause appears to be divergent, with lower risk of heart disease when started within five years, but no impact after ten.[23][24][25] There may be an increase in heart disease if HRT is given twenty years post-menopause.[26] This variability has led some reviews to suggest an absence of significant effect on morbidity.[27] Importantly, there is no difference in long-term mortality from HRT, regardless of age.[6]
A Cochrane review suggested that women starting HRT less than 10 years after menopause had lower mortality and coronary heart disease, without any strong effect on the risk of stroke and pulmonary embolism.[23] Those starting therapy more than 10 years after menopause showed little effect on mortality and coronary heart disease, but an increased risk of stroke. Both therapies had an association with venous clots and pulmonary embolism.[23]
HRT with estrogen and progesterone also improves cholesterol levels. With menopause, HDL decreases, while LDL, triglycerides and lipoprotein a increase, patterns that reverse with estrogen. Beyond this, HRT improves heart contraction, coronary blood flow, sugar metabolism, and decreases platelet aggregation and plaque formation. HRT may promote reverse cholesterol transport through induction of cholesterol ABC transporters.[28] HRT also results in a large reduction in the pro-thrombotic lipoprotein a.[29] Studies on cardiovascular disease with testosterone therapy have been mixed, with some suggesting no effect or a mild negative effect, though others have shown an improvement in surrogate markers such as cholesterol, triglycerides and weight.[30][31] Testosterone has a positive effect on vascular endothelial function and tone with observational studies suggesting that women with lower testosterone may be at greater risk for heart disease. Available studies are limited by small sample size and study design. Low sex hormone binding globulin, which occurs with menopause, is associate with increased body mass index and risk for type 2 diabetes.[32]
Blood clots
Effects of hormone replacement therapy on venous blood clot formation and potential for pulmonary embolism may vary with different estrogen and progestogen therapies, and with different doses or method of use.[18] Comparisons between routes of administration suggest that when estrogens are applied to the skin or vagina, there is a lower risk of blood clots,[18][33] whereas when used orally, the risk of blood clots and pulmonary embolism is increased.[23] Skin and vaginal routes of hormone therapy are not subject to first pass metabolism, and so lack the anabolic effects that oral therapy has on liver synthesis of vitamin K-dependent clotting factors, possibly explaining why oral therapy may increase blood clot formation.[34]
While a 2018 review found that taking progesterone and estrogen together can decrease this risk,[33] other reviews reported an increased risk of blood clots and pulmonary embolism when estrogen and progestogen were combined, particularly when treatment was started 10 years or more after menopause and when the women were older than 60 years.[18][23]
The risk of venous thromboembolism may be reduced with bioidentical preparations, though research on this is only preliminary.[35]
| v]] · [[Template talk:Risk of venous thromboembolism with hormone therapy and birth control pills (QResearch/CPRD) | d]] · e
Risk of venous thromboembolism (VTE) with hormone therapy and birth control (QResearch/CPRD) | ||
| Type | Route | Medications | Odds ratio (95% CI) |
|---|---|---|---|
| Menopausal hormone therapy | Oral | Estradiol alone ≤1 mg/day >1 mg/day |
1.27 (1.16–1.39)* 1.22 (1.09–1.37)* 1.35 (1.18–1.55)* |
| Conjugated estrogens alone ≤0.625 mg/day >0.625 mg/day |
1.49 (1.39–1.60)* 1.40 (1.28–1.53)* 1.71 (1.51–1.93)* | ||
| Estradiol/medroxyprogesterone acetate | 1.44 (1.09–1.89)* | ||
| Estradiol/dydrogesterone ≤1 mg/day E2 >1 mg/day E2 |
1.18 (0.98–1.42) 1.12 (0.90–1.40) 1.34 (0.94–1.90) | ||
| Estradiol/norethisterone ≤1 mg/day E2 >1 mg/day E2 |
1.68 (1.57–1.80)* 1.38 (1.23–1.56)* 1.84 (1.69–2.00)* | ||
| Estradiol/norgestrel or estradiol/drospirenone | 1.42 (1.00–2.03) | ||
| Conjugated estrogens/medroxyprogesterone acetate | 2.10 (1.92–2.31)* | ||
| Conjugated estrogens/norgestrel ≤0.625 mg/day CEEs >0.625 mg/day CEEs |
1.73 (1.57–1.91)* 1.53 (1.36–1.72)* 2.38 (1.99–2.85)* | ||
| Tibolone alone | 1.02 (0.90–1.15) | ||
| Raloxifene alone | 1.49 (1.24–1.79)* | ||
| Transdermal | Estradiol alone ≤50 μg/day >50 μg/day |
0.96 (0.88–1.04) 0.94 (0.85–1.03) 1.05 (0.88–1.24) | |
| Conjugated estrogens alone | 1.04 (0.76–1.43) | ||
| Estradiol/progestogen | 0.88 (0.73–1.01) | ||
| Vaginal | Estradiol alone | 0.84 (0.73–0.97) | |
| Combined birth control | Oral | Ethinylestradiol/norethisterone | 2.56 (2.15–3.06)* |
| Ethinylestradiol/levonorgestrel | 2.38 (2.18–2.59)* | ||
| Ethinylestradiol/norgestimate | 2.53 (2.17–2.96)* | ||
| Ethinylestradiol/desogestrel | 4.28 (3.66–5.01)* | ||
| Ethinylestradiol/gestodene | 3.64 (3.00–4.43)* | ||
| Ethinylestradiol/drospirenone | 4.12 (3.43–4.96)* | ||
| Ethinylestradiol/cyproterone acetate | 4.27 (3.57–5.11)* | ||
| Notes: (1) Nested case–control studies (2015, 2019) based on data from the QResearch and Clinical Practice Research Datalink (CPRD) databases. (2) Bioidentical progesterone was not included, but is known to be associated with no additional risk. Footnotes: * = Statistically significant (p < 0.01). Sources: See template. | |||
Stroke
Multiple studies suggest that the possibility of HRT related stroke is absent if therapy is started within five years of menopause,[36] and that the association is absent or even preventive when given by non-oral routes.[8] Ischemic stroke risk was increased during the time of intervention in the WHI, with no significant effect after the cessation of therapy[26] and no difference in mortality at long term follow up.[6] When oral synthetic estrogen or combined estrogen-progestogen treatment is delayed until five years from menopause, cohort studies in Swedish women have suggested an association with hemorrhagic and ischemic stroke.[36] Another large cohort of Danish women suggested that the specific route of administration was important, finding that although oral estrogen increased risk of stroke, absorption through the skin had no impact, and vaginal estrogen actually had a decreased risk.[8]
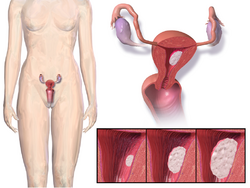
Endometrial cancer
In postmenopausal women, continuous combined estrogen plus progestin decreases endometrial cancer incidence.[37] The duration of progestogen therapy should be at least 14 days per cycle to prevent endometrial disease.[38]
Endometrial cancer has been grouped into two forms in the context of hormone replacement. Type 1 is the most common, can be associated with estrogen therapy, and is usually low grade. Type 2 is not related to estrogen stimulation and usually higher grade and poorer in prognosis.[39] The endometrial hyperplasia that leads to endometrial cancer with estrogen therapy can be prevented by concomitant administration of progestogen.[39] The extensive use of high-dose estrogens for birth control in the 1970s is thought to have resulted in a significant increase in the incidence of type 1 endometrial cancer.[40]
Paradoxically, progestogens do promote the growth of uterine fibroids, and a pelvic ultrasound can be performed before beginning HRT to make sure there are no underlying uterine or endometrial lesions.[39]
Androgens do not stimulate endometrial proliferation in post menopausal women, and appear to inhibit the proliferation induced by estrogen to a certain extent.[41]
There is insufficient high‐quality evidence to inform women considering hormone replacement therapy after treatment for endometrial cancer.[42]
Breast cancer
Studies regarding the association of breast cancer with hormone replacement are inconsistent and vary with type of replacement and time since menopause. While some evaluations suggest an increased risk, in others it is decreased.[43] This inconsistency of effect has been suggested to imply a lack of meaningful impact of HRT on breast cancer risk.[27]
There is a non-statistically significant increased rate of breast cancer for hormone replacement therapy with synthetic progesterone.[6] The risk may be reduced with bioidentical progesterone,[44] though the only prospective study that suggested this was underpowered due to the rarity of breast cancer in the control population. There have been no randomized controlled trials as of 2018.[43] The relative risk of breast cancer also varies depending on the interval between menopause and HRT and route of synthetic progestin administration.[45][46]
The most recent follow up of the Women's Health Initiative participants demonstrated a lower incidence of breast cancer in post-hysterectomy participants taking equine estrogen alone, though the relative risk was increased if estrogen was taken with medroxy-progesterone.[47] Estrogen is usually only given alone in the setting of a hysterectomy due to the increased risk of vaginal bleeding and uterine cancer with unopposed estrogen.[48][49]
HRT has been more strongly associated with risk of breast cancer in women with lower body mass indices (BMIs). No breast cancer association has been found with BMIs of over 25.[50] It has been suggested by some that the absence of significant effect in some of these studies could be due to selective prescription to overweight women who have higher baseline estrone, or to the very low progesterone serum levels after oral administration leading to a high tumor inactivation rate.[51]
Evaluating the response of breast tissue density to HRT using mammography appears to help assessing the degree of breast cancer risk associated with therapy; women with dense or mixed-dense breast tissue have a higher risk of developing breast cancer than those with low density tissue.[52]
Micronized progesterone does not appear to be associated with breast cancer risk when used for less than five years with limited data suggesting an increased risk when used for longer duration.[53]
A retrospective Cox proportional hazards analysis from Richard Neapolitan endorsed the reduced risk breast cancer with conjugated equine estrogen usage alone, but also suggested that conjugated equine estrogen with medroxyprogesterone acetate was associated with a risk reduction in breast cancer and that bioidentical hormone therapy was not associated with a statistically significant effect.[43] There have been no prospective randomized clinical trials comparing the two routes of administration with regards to breast cancer.[54]
For women who previously have had breast cancer, it is recommended to first consider other options for menopausal effects, such as bisphosphonates or selective estrogen receptor modulators (SERMs) for osteoporosis, cholesterol-lowering agents and aspirin for cardiovascular disease, and vaginal estrogen for local symptoms. Observational studies of systemic HRT after breast cancer are generally reassuring. If HRT is necessary after breast cancer, estrogen-only therapy or estrogen therapy with a progestogen may be safer options than combined systemic therapy.[55] In women who are BRCA1 or BRCA2 mutation carriers, HRT does not appear to impact breast cancer risk.[56] The relative number of women using HRT who also obtain regular screening mammograms is higher than that in women who do not use HRT, a factor which has been suggested as contributing to different breast cancer detection rates in the two groups.[57]
With androgen therapy, pre-clinical studies have suggested an inhibitory effect on breast tissue though the majority of epidemiological studies suggest a positive association.[58]
Ovarian cancer
HRT is associated with an increased risk of ovarian cancer, with women using HRT having about one additional case of ovarian cancer per 1,000 users.[59] This risk is decreased when progestogen therapy is given concomitantly, as opposed to estrogen alone, and also decreases with increasing time since stopping HRT.[60] Regarding the specific subtype, there may be a higher risk of serous cancer, but no association with clear cell, endometrioid, or mucinous ovarian cancer.[60] Hormonal therapy in ovarian cancer survivors after surgical removal of the ovaries is generally thought to improval survival rates.[61]
Other malignancies
Colorectal cancer
In the WHI, women who took combined estrogen-progesterone therapy had a lower risk of getting colorectal cancer. However, the cancers they did have were more likely to have spread to lymph nodes or distant sites than colorectal cancer in women not taking hormones.[62] In colorectal cancer survivors, usage of HRT is thought to lead to lower recurrence risk and overall mortality.[63]
Cervical cancer
There appears to be a significantly decreased risk of cervical squamous cell cancer in post menopausal women treated with HRT and a weak increase in adenocarcinoma. No studies have reported an increased risk of recurrence when HRT is used with cervical cancer survivors.[64]
Sexual function
HRT can help with the lack of sexual desire and sexual dysfunction that can occur with menopause. Epidemiological surveys of women between 40 and 69 years suggest that 75% of women remain sexually active after menopause.[4] With increasing life spans, women today are living one third or more of their lives in a postmenopausal state, a period during which healthy sexuality can be integral to their quality of life.[65]
Decreased libido and sexual dysfunction are common issues in postmenopausal women, an entity referred to hypoactive sexual desire disorder (HSDD); its signs and symptoms can both be improved by HRT.[5][66] Several hormonal changes take place during this period, including a decrease in estrogen and an increase in follicle-stimulating hormone. For most women, the majority of change occurs during the late perimenopausal and postmenopausal stages.[4] Decreases in sex hormone-binding globulin (SHBG) and inhibin (A and B) also occur. Testosterone is present in women at a lower level than men, peaking at age 30 and declining gradually with age; there is less variation during the menopausal transition relative to estrogen and progesterone.[4]
A global consensus position statement has advised that postmenopausal testosterone replacement to premenopausal levels can be effective for HSDD. Safety information for testosterone treatment is not available beyond two years of continuous therapy however and dosing above physiologic levels is not advised.[31] Testosterone patches have been found to restore sexual desire in post menopausal women.[67] There is insufficient data to evaluate the impact of testosterone replacement on heart disease, breast cancer, with most trials having included women taking concomitant estrogen and progesterone and with testosterone therapy itself being relatively short in duration. In the setting of this limited data, testosterone therapy has not been associated with adverse events.[31]
Not all women are responsive, especially those with preexisting sexual difficulties.[22] Estrogen replacement can restore vaginal cells, pH levels, and blood flow to the vagina, all of which tend to deteriorate at the onset of menopause. Pain or discomfort with sex appears to be the most responsive component to estrogen.[22] It also has been shown to have positive effects on the urinary tract.[22] Estrogen can also reduce vaginal atrophy and increase sexual arousal, frequency and orgasm.[22]
The effectiveness of hormone replacement can decline in some women after long-term use.[22] A number of studies have also found that the combined effects of estrogen/androgen replacement therapy can increase libido and arousal over estrogen alone.[22] Tibolone, a synthetic steroid with estrogenic, androgenic, and progestogenic properties that is available in Europe, has the ability to improve mood, libido, and physical symptomatology. In various placebo-controlled studies, improvements in vasomotor symptoms, emotional response, sleep disturbances, physical symptoms, and sexual desire have been seen, though it also carries a similar risk profile to conventional HRT.[5]
Neurodegenerative disorders
For prevention, the WHI suggested that HRT may increase risk of dementia if initiated after 65 years of age, but have a neutral outcome or be neuroprotective for those between 50 and 55 years.[26] Other studies in perimenopause have shown HRT to be consistently associated with a lower risk of Alzheimer's.[68][69] With Parkinson's the majority of clinical and epidemiological studies show have demonstrated either no association[70][71] or inconclusive results.[68][72] A Danish study suggested an increased risk of Parkinson's with HRT in cyclical dosing schedules.[73]
With regards to treatment, randomized trials have shown that HRT improves executive and attention processes outside of the context of dementia in postmenopausal women, both in those that are asymptomatic and those with mild cognitive impairment.[74][75][76] Estrogen replacement appears to improve motor symptoms and activities of daily living in post menopausal women with Parkinson's, with significant improvement of UPDRS scores.[77] Clinical trials have also shown testosterone replacement to be associated with small statistically significant improvements in verbal learning and memory in postmenopausal women.[78] DHEA has not been found to improve cognitive performance after menopause.[32] Pre-clinical studies indicate that endogenous estrogen and testosterone are neuroprotective and can prevent brain amyloid deposition.[79][80]
Muscle and bone
There is a significant decrease in hip fracture risk during treatment that to a lesser degree persists after HRT is stopped.[26][81] It also helps collagen formation, which in turn improves intervertebral disc and bone strength.[82]
Hormone replacement therapy in the form of estrogen and androgen can be effective at reversing the effects of aging on muscle.[83] Lower testosterone is associated with lower bone density and higher free testosterone is associated with lower hip fracture rates in older women.[32] Testosterone therapy, which can be used for decreased sexual function, can also increase bone mineral density and muscle mass.[31]
Side effects
Side effects in HRT occur with varying frequency and include:[84]
Common
- Headache
- Upset stomach, stomach cramps or bloating
- Diarrhea
- Appetite and weight changes
- Changes in sex drive or performance
- Nervousness
- Brown or black patches on the skin
- Acne
- Swelling of hands, feet, or lower legs due to fluid retention
- Changes in menstrual flow
- Breast tenderness, enlargement, or discharge
- Sudden difficulty wearing contact lenses
Uncommon
- Double vision
- Severe abdominal pain
- Yellowing of skin or eyes
- Severe depression
- Unusual bleeding
- Loss of appetite
- Skin rash
- Lassitude
- Fever
- Dark-colored urine
- Light colored stool
- Chorea[85]
Contraindications
The following are absolute and relative contraindications to HRT:[86]
Absolute contraindications
- Undiagnosed vaginal bleeding
- Severe liver disease
- Pregnancy
- Severe coronary artery disease
- Aggressive breast, uterine or ovarian cancer
Relative contraindications
- Migraine headaches
- History of breast cancer
- History of ovarian cancer
- Venous thrombosis
- History of uterine fibroids
- Atypical ductal hyperplasia of the breast
- Active gallbladder disease (cholangitis, cholecystitis)
- Well-differentiated and early endometrial cancer – once treatment for the malignancy is complete, is no longer an absolute contraindication.
History and research
The extraction of CEEs from the urine of pregnant mares led to the marketing in 1942 of Premarin, one of the earlier forms of estrogen to be introduced.[87][88] From that time until the mid-1970s, estrogen was administered without a supplemental progestogen. Beginning in 1975, studies began to show that without a progestogen, unopposed estrogen therapy with Premarin resulted in an eight-fold increased risk of endometrial cancer, eventually causing sales of Premarin to plummet.[87] It was recognized in the early 1980s that the addition of a progestogen to estrogen reduced this risk to the endometrium.[87] This led to the development of combined estrogen–progestogen therapy, most commonly with a combination of conjugated equine estrogen (Premarin) and medroxyprogesterone (Provera).[87]
Trials
The Women's Health Initiative trials were conducted between 1991 and 2006 and were the first large, double-blind, placebo-controlled clinical trials of HRT in healthy women.[87] Their results were both positive and negative, suggesting that during the time of hormone therapy itself, there are increases in invasive breast cancer, stroke and lung clots. Other risks include increased endometrial cancer, gallbladder disease, and urinary incontinence, while benefits include decreased hip fractures, decreased incidence of diabetes, and improvement of vasomotor symptoms. There is also an increased risk of dementia with HRT in women over 65, though at younger ages it appears to be neuroprotective. After the cessation of HRT, the WHI continued to observe its participants, and found that most of these risks and benefits dissipated, though some elevation in breast cancer risk did persist.[26] Other studies have also suggested an increased risk of ovarian cancer.[60]
The arm of the WHI receiving combined estrogen and progestin therapy was closed prematurely in 2002 by its Data Monitoring Committee (DMC) due to perceived health risks, though this occurred a full year after the data suggesting increased risk became manifest. In 2004, the arm of the WHI in which post-hysterectomy patients were being treated with estrogen alone was also closed by the DMC. Clinical medical practice changed based upon two parallel Women's Health Initiative (WHI) studies of HRT. Prior studies were smaller, and many were of women who electively took hormonal therapy. One portion of the parallel studies followed over 16,000 women for an average of 5.2 years, half of whom took placebo, while the other half took a combination of CEEs and MPA (Prempro). This WHI estrogen-plus-progestin trial was stopped prematurely in 2002 because preliminary results suggested risks of combined CEEs and progestins exceeded their benefits. The first report on the halted WHI estrogen-plus-progestin study came out in July 2002.[89]
Initial data from the WHI in 2002 suggested mortality to be lower when HRT was begun earlier, between age 50 to 59, but higher when begun after age 60. In older patients, there was an apparent increased incidence of breast cancer, heart attacks, venous thrombosis, and stroke, although a reduced incidence of colorectal cancer and bone fracture. At the time, The WHI recommended that women with non-surgical menopause take the lowest feasible dose of HRT for the shortest possible time to minimize associated risks.[89] Some of the WHI findings were again found in a larger national study done in the United Kingdom, known as the Million Women Study (MWS). As a result of these findings, the number of women taking HRT dropped precipitously.[90] In 2012, the United States Preventive Task Force (USPSTF) concluded that the harmful effects of combined estrogen and progestin therapy likely exceeded their chronic disease prevention benefits.[91][92]
In 2002 when the first WHI follow up study was published, with HRT in post menopausal women, both older and younger age groups had a slightly higher incidence of breast cancer, and both heart attack and stroke were increased in older patients, although not in younger participants. Breast cancer was increased in women treated with estrogen and a progestin, but not with estrogen and progesterone or estrogen alone. Treatment with unopposed estrogen (i.e., an estrogen alone without a progestogen) is contraindicated if the uterus is still present, due to its proliferative effect on the endometrium. The WHI also found a reduced incidence of colorectal cancer when estrogen and a progestogen were used together, and most importantly, a reduced incidence of bone fractures. Ultimately, the study found disparate results for all cause mortality with HRT, finding it to be lower when HRT was begun during ages 50–59, but higher when begun after age 60. The authors of the study recommended that women with non-surgical menopause take the lowest feasible dose of hormones for the shortest time to minimize risk.[89]
The data published by the WHI suggested supplemental estrogen increased risk of venous thromboembolism and breast cancer but was protective against osteoporosis and colorectal cancer, while the impact on cardiovascular disease was mixed.[93] These results were later supported in trials from the United Kingdom, but not in more recent studies from France and China. Genetic polymorphism appears to be associated with inter-individual variability in metabolic response to HRT in postmenopausal women.[94][95]
| Clinical outcome | Hypothesized effect on risk |
Estrogen and progestogen (CEs 0.625 mg/day p.o. + MPA 2.5 mg/day p.o.) (n = 16,608, with uterus, 5.2–5.6 years follow up) |
Estrogen alone (CEs 0.625 mg/day p.o.) (n = 10,739, no uterus, 6.8–7.1 years follow up) | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | AR | HR | 95% CI | AR | ||
| Coronary heart disease | Decreased | 1.24 | 1.00–1.54 | +6 / 10,000 PYs | 0.95 | 0.79–1.15 | −3 / 10,000 PYs |
| Stroke | Decreased | 1.31 | 1.02–1.68 | +8 / 10,000 PYs | 1.37 | 1.09–1.73 | +12 / 10,000 PYs |
| Pulmonary embolism | Increased | 2.13 | 1.45–3.11 | +10 / 10,000 PYs | 1.37 | 0.90–2.07 | +4 / 10,000 PYs |
| Venous thromboembolism | Increased | 2.06 | 1.57–2.70 | +18 / 10,000 PYs | 1.32 | 0.99–1.75 | +8 / 10,000 PYs |
| Breast cancer | Increased | 1.24 | 1.02–1.50 | +8 / 10,000 PYs | 0.80 | 0.62–1.04 | −6 / 10,000 PYs |
| Colorectal cancer | Decreased | 0.56 | 0.38–0.81 | −7 / 10,000 PYs | 1.08 | 0.75–1.55 | +1 / 10,000 PYs |
| Endometrial cancer | – | 0.81 | 0.48–1.36 | −1 / 10,000 PYs | – | – | – |
| Hip fractures | Decreased | 0.67 | 0.47–0.96 | −5 / 10,000 PYs | 0.65 | 0.45–0.94 | −7 / 10,000 PYs |
| Total fractures | Decreased | 0.76 | 0.69–0.83 | −47 / 10,000 PYs | 0.71 | 0.64–0.80 | −53 / 10,000 PYs |
| Total mortality | Decreased | 0.98 | 0.82–1.18 | −1 / 10,000 PYs | 1.04 | 0.91–1.12 | +3 / 10,000 PYs |
| Global index | – | 1.15 | 1.03–1.28 | +19 / 10,000 PYs | 1.01 | 1.09–1.12 | +2 / 10,000 PYs |
| Diabetes | – | 0.79 | 0.67–0.93 | 0.88 | 0.77–1.01 | ||
| Gallbladder disease | Increased | 1.59 | 1.28–1.97 | 1.67 | 1.35–2.06 | ||
| Stress incontinence | – | 1.87 | 1.61–2.18 | 2.15 | 1.77–2.82 | ||
| Urge incontinence | – | 1.15 | 0.99–1.34 | 1.32 | 1.10–1.58 | ||
| Peripheral artery disease | – | 0.89 | 0.63–1.25 | 1.32 | 0.99–1.77 | ||
| Probable dementia | Decreased | 2.05 | 1.21–3.48 | 1.49 | 0.83–2.66 | ||
| Abbreviations: CEs = conjugated estrogens. MPA = medroxyprogesterone acetate. p.o. = per oral. HR = hazard ratio. AR = attributable risk. PYs = person–years. CI = confidence interval. Notes: Sample sizes (n) include placebo recipients, which were about half of patients. "Global index" is defined for each woman as the time to earliest diagnosis for coronary heart disease, stroke, pulmonary embolism, breast cancer, colorectal cancer, endometrial cancer (estrogen plus progestogen group only), hip fractures, and death from other causes. Sources: See template. | |||||||
The WHI reported statistically significant increases in rates of breast cancer, coronary heart disease, strokes and pulmonary emboli. The study also found statistically significant decreases in rates of hip fracture and colorectal cancer. "A year after the study was stopped in 2002, an article was published indicating that estrogen plus progestin also increases the risks of dementia."[96] The conclusion of the study was that the HRT combination presented risks that outweighed its measured benefits. The results were almost universally reported as risks and problems associated with HRT in general, rather than with Prempro, the specific proprietary combination of CEEs and MPA studied.
After the increased clotting found in the first WHI results was reported in 2002, the number of Prempro prescriptions filled reduced by almost half. Following the WHI results, a large percentage of HRT users opted out of them, which was quickly followed by a sharp drop in breast cancer rates. The decrease in breast cancer rates has continued in subsequent years.[97] An unknown number of women started taking alternatives to Prempro, such as compounded bioidentical hormones, though researchers have asserted that compounded hormones are not significantly different from conventional hormone therapy.[98]
The other portion of the parallel studies featured women who were post hysterectomy and so received either placebo progestogen or CEEs alone. This group did not show the risks demonstrated in the combination hormone study, and the estrogen-only study was not halted in 2002. However, in February 2004 it, too, was halted. While there was a 23% decreased incidence of breast cancer in the estrogen-only study participants, risks of stroke and pulmonary embolism were increased slightly, predominantly in patients who began HRT over the age of 60.[99]
Several other large studies and meta-analyses have reported reduced mortality for HRT in women younger than age 60 or within 10 years of menopause, and a debatable or absent effect on mortality in women over 60.[100][101][102][103][17][104]
Though research thus far has been substantial, further investigation is needed to fully understand differences in effect for different types of HRT and lengths of time since menopause.[105][106][82] (As of 2023), for example, no trial has studied women who begin taking HRT around age 50 and continue taking it for longer than 10 years.[107]
Available forms
There are five major human steroid hormones: estrogens, progestogens, androgens, mineralocorticoids, and glucocorticoids. Estrogens and progestogens are the two most often used in menopause. They are available in a wide variety of FDA approved and non–FDA-approved formulations.[9]
In women with intact uteruses, estrogens are almost always given in combination with progestogens, as long-term unopposed estrogen therapy is associated with a markedly increased risk of endometrial hyperplasia and endometrial cancer.[1][2] Conversely, in women who have undergone a hysterectomy or do not have a uterus, a progestogen is not required, and estrogen can be used alone. There are many combined formulations which include both estrogen and progestogen.
Specific types of hormone replacement include:[1][2]
- Estrogens – bioidentical estrogens like estradiol and estriol, animal-derived estrogens like conjugated estrogens (CEEs), and synthetic estrogens like ethinylestradiol
- Progestogens – bioidentical progesterone, and progestins (synthetic progestogens) like medroxyprogesterone acetate (MPA), norethisterone, and dydrogesterone
- Androgens – bioidentical testosterone and dehydroepiandrosterone (DHEA), and synthetic anabolic steroids like methyltestosterone and nandrolone decanoate[108][109]
Tibolone – a synthetic medication available in Europe but not the United States– is more effective than placebo but less effective than combination hormone therapy in postmenopausal women. It may have a decreased risk of breast and colorectal cancer, though conversely it can be associated with vaginal bleeding, endometrial cancer, and increase the risk of stroke in women over age 60 years.[110][111]
Vaginal estrogen can improve local atrophy and dryness, with fewer systemic effects than estrogens delivered by other routes.[112] Sometimes an androgen, generally testosterone, can be added to treat diminished libido.[113][114]
Continuous versus cyclic
Dosage is often varied cyclically to more closely mimic the ovarian hormone cycle, with estrogens taken daily and progestogens taken for about two weeks every month or every other month, a schedule referred to as 'cyclic' or 'sequentially combined'. Alternatively, 'continuous combined' HRT can be given with a constant daily hormonal dosage.[115] Continuous combined HRT is associated with less complex endometrial hyerplasia than cyclic.[116] Impact on breast density appears to be similar in both regimen timings.[117]
Route of administration
The medications used in menopausal HRT are available in numerous different formulations for use by a variety of different routes of administration:[1][2]
- Oral administration – tablets, capsules
- Transdermal administration – patches, gels, creams
- Vaginal administration – tablets, creams, suppositories, rings
- Intramuscular or subcutaneous injection – solutions in vials or ampoules
- Subcutaneous implant – surgically-inserted pellets placed into fat tissue
- Less commonly sublingual, buccal, intranasal, and rectal administration, as well as intrauterine devices
More recently developed forms of drug delivery are alleged to have increased local effect lower dosing, fewer side effects, and constant rather than cyclical serum hormone levels.[1][2] Transdermal and vaginal estrogen, in particular, avoid first pass metabolism through the liver. This in turn prevents an increase in clotting factors and accumulation of anti-estrogenic metabolites, resulting in fewer adverse side effects, particularly with regard to cardiovascular disease and stroke.[118]
Injectable forms of estradiol exist and have been used occasionally in the past.[119][120] However, they are rarely used in menopausal hormone therapy in modern times and are no longer recommended.[119][121] Instead, other non-oral forms of estradiol such as transdermal estradiol are recommended and may be used.[119] Estradiol injectables are generally well-tolerated and convenient, requiring infrequent administration.[119][120] However, this form of estradiol does not release estradiol at a constant rate and there are very high circulating estradiol levels soon after injection followed by a rapid decline in levels.[119] Injections may also be painful.[119] Examples of estradiol injectables that may be used in menopausal hormone therapy include estradiol valerate and estradiol cypionate.[119][120] In terms of injectable progestogens, injectable progesterone is associated with pain and injection site reactions as well as a short duration of action requiring very frequent injections, and is similarly not recommended in menopausal hormone therapy.[122][120]
Bioidentical hormone therapy
Bioidentical hormone therapy (BHT) is the usage of hormones that are chemically identical to those produced in the body. Although proponents of BHT claim advantages over non-bioidentical or conventional hormone therapy, the FDA does not recognize the term 'bioidentical hormone', stating there is no scientific evidence that these hormones are identical to their naturally occurring counterparts.[11][123] There are, however, FDA approved products containing hormones classified as 'bioidentical'.[12][9]
Bioidentical hormones can be used in either pharmaceutical or compounded preparations, with the latter generally not recommended by regulatory bodies due to their lack of standardization and regulatory oversight.[11] Most classifications of bioidentical hormones do not take into account manufacturing, source, or delivery method of the products, and so describe both non-FDA approved compounded products and FDA approved pharmaceuticals as 'bioidentical'.[9] The British Menopause Society has issued a consensus statement endorsing the distinction between "compounded" forms (cBHRT), described as unregulated, custom made by specialty pharmacies and subject to heavy marketing and "regulated" pharmaceutical grade forms (rBHRT), which undergo formal oversight by entities such as the FDA and form the basis of most clinical trials.[54] Some practitioners recommending compounded bioidentical HRT also use salivary or serum hormonal testing to monitor response to therapy, a practice not endorsed by current clinical guidelines in the United States and Europe.[124]
Bioidentical hormones in pharmaceuticals may have very limited clinical data, with no randomized controlled prospective trials to date comparing them to their animal derived counterparts. Some pre-clinical data has suggested a decreased risk of venous thromboembolism, cardiovascular disease, and breast cancer.[11] As of 2012, guidelines from the North American Menopause Society, the Endocrine Society, the International Menopause Society, and the European Menopause and Andropause Society endorsed the reduced risk of bioidentical pharmaceuticals for those with increased clotting risk.[11][125]
Compounding
Compounding for HRT is generally discouraged by the FDA and medical industry in the United States due to a lack of regulation and standardized dosing.[11][123] The U.S. Congress did grant the FDA explicit but limited oversight of compounded drugs in a 1997 amendment to the Federal Food, Drug, and Cosmetic Act (FDCA), but they have encountered obstacles in this role since that time. After 64 patient deaths and 750 harmed patients from a 2012 meningitis outbreak due to contaminated steroid injections, Congress passed the 2013 Drug Quality and Security Act, authorizing creation by the FDA of a voluntary registration for facilities that manufactured compounded drugs, and reinforcing FDCA regulations for traditional compounding.[126] The DQSA and its reinforcement of provision §503A of the FDCA solidifies FDA authority to enforce FDCA regulation of against compounders of bioidentical hormone therapy.[126]
In the United Kingdom, on the other hand, compounding is a regulated activity. The Medicines and Healthcare products Regulatory Agency regulates compounding performed under a Manufacturing Specials license and the General Pharmaceutical Council regulates compounding performed within a pharmacy. All testosterone prescribed in the United Kingdom is bioidentical, with its use supported by the National Health Service. There is also marketing authorisation for male testosterone products. National Institute for Health and Care Excellence guideline 1.4.8 states: "consider testosterone supplementation for menopausal women with low sexual desire if HRT alone is not effective". The footnote adds: "at the time of publication (November 2015), testosterone did not have a United Kingdom marketing authorisation for this indication in women. Bioidentical progesterone is used in IVF treatment and for pregnant women who are at risk of premature labour."
Society and public perception
Wyeth controversy
Wyeth, now a subsidiary of Pfizer, was a pharmaceutical company that marketed the HRT products Premarin (CEEs) and Prempro (CEEs + MPA).[127][128] In 2009, litigation involving Wyeth resulted in the release of 1,500 documents that revealed practices concerning its promotion of these medications.[127][128][129] The documents showed that Wyeth commissioned dozens of ghostwritten reviews and commentaries that were published in medical journals to promote unproven benefits of its HRT products, downplay their harms and risks, and cast competing therapies in a negative light.[127][128][129] Starting in the mid-1990s and continuing for over a decade, Wyeth pursued an aggressive "publication plan" strategy to promote its HRT products through the use of ghostwritten publications.[129] It worked mainly with DesignWrite, a medical writing firm.[129] Between 1998 and 2005, Wyeth had 26 papers promoting its HRT products published in scientific journals.[127]
These favorable publications emphasized the benefits and downplayed the risks of its HRT products, especially the "misconception" of the association of its products with breast cancer.[129] The publications defended unsupported cardiovascular "benefits" of its products, downplayed risks such as breast cancer, and promoted off-label and unproven uses like prevention of dementia, Parkinson's disease, vision problems, and wrinkles.[128] In addition, Wyeth emphasized negative messages against the SERM raloxifene for osteoporosis, instructed writers to stress the fact that "alternative therapies have increased in usage since the WHI even though there is little evidence that they are effective or safe...", called into question the quality and therapeutic equivalence of approved generic CEE products, and made efforts to spread the notion that the unique risks of CEEs and MPA were a class effect of all forms of menopausal HRT: "Overall, these data indicate that the benefit/risk analysis that was reported in the Women's Health Initiative can be generalized to all postmenopausal hormone replacement therapy products."[128]
Following the publication of the WHI data in 2002, the stock prices for the pharmaceutical industry plummeted, and huge numbers of women stopped using HRT.[130] The stocks of Wyeth, which supplied the Premarin and Prempro that were used in the WHI trials, decreased by more than 50%, and never fully recovered.[130] Some of their articles in response promoted themes such as the following: "the WHI was flawed; the WHI was a controversial trial; the population studied in the WHI was inappropriate or was not representative of the general population of menopausal women; results of clinical trials should not guide treatment for individuals; observational studies are as good as or better than randomized clinical trials; animal studies can guide clinical decision-making; the risks associated with hormone therapy have been exaggerated; the benefits of hormone therapy have been or will be proven, and the recent studies are an aberration."[87] Similar findings were observed in a 2010 analysis of 114 editorials, reviews, guidelines, and letters by five industry-paid authors.[87] These publications promoted positive themes and challenged and criticized unfavorable trials such as the WHI and MWS.[87] In 2009, Wyeth was acquired by Pfizer in a deal valued at US$68 billion.[131][132] Pfizer, a company that produces Provera and Depo-Provera (MPA) and has also engaged in medical ghostwriting, continues to market Premarin and Prempro, which remain best-selling medications.[87][129]
According to Fugh-Berman (2010), "Today, despite definitive scientific data to the contrary, many gynecologists still believe that the benefits of [HRT] outweigh the risks in asymptomatic women. This non-evidence–based perception may be the result of decades of carefully orchestrated corporate influence on medical literature."[128] As many as 50% of physicians have expressed skepticism about large trials like the WHI and HERS.[133] The positive perceptions of many physicians of HRT in spite of large trials showing risks that potentially outweigh any benefits may be due to the efforts of pharmaceutical companies like Wyeth.[129][87]
Popularity
The 1990s showed a dramatic decline in prescription rates, though more recently they have begun to rise again.[118][134] Transdermal therapy, in part due to its lack of increase in venous thromboembolism, is now often the first choice for HRT in the United Kingdom. Conjugate equine estrogen, in distinction, has a potentially higher thrombosis risk and is now not commonly used in the UK, replaced by estradiol based compounds with lower thrombosis risk. Oral progestogen combinations such as medroxyprogesterone acetate have changed to dyhydrogesterone, due to a lack of association of the latter with venous clot.[135]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline". J. Clin. Endocrinol. Metab. 100 (11): 3975–4011. November 2015. doi:10.1210/jc.2015-2236. PMID 26444994. http://eprints.gla.ac.uk/113696/1/113696.pdf.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Postmenopausal hormone therapy: an Endocrine Society scientific statement". J. Clin. Endocrinol. Metab. 95 (7 Suppl 1): s1–s66. July 2010. doi:10.1210/jc.2009-2509. PMID 20566620.
- ↑ Shuster, Lynne T.; Rhodes, Deborah J.; Gostout, Bobbie S.; Grossardt, Brandon R.; Rocca, Walter A. (2010). "Premature menopause or early menopause: Long-term health consequences". Maturitas 65 (2): 161–166. doi:10.1016/j.maturitas.2009.08.003. ISSN 0378-5122. PMID 19733988.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "Quality of sexual life and menopause". Women's Health 6 (4): 385–396. 1 July 2009. doi:10.2217/WHE.09.24. PMID 19586430.
- ↑ 5.0 5.1 5.2 Ziaei S., Moghasemi M., Faghihzadeh S. (2010). "Comparative effects of conventional hormone replacement therapy and tibolone on climacteric symptoms and sexual dysfunction in postmenopausal women". Climacteric 13 (3): 147–156. doi:10.1016/j.maturitas.2006.04.014. PMID 16730929.
- ↑ 6.0 6.1 6.2 6.3 Manson, JE; Aragaki, AK; Rossouw, JE; Anderson, GL; Prentice, RL; LaCroix, AZ; Chlebowski, RT; Howard, BV et al. (12 September 2017). "Menopausal Hormone Therapy and Long-term All-Cause and Cause-Specific Mortality: The Women's Health Initiative Randomized Trials.". JAMA 318 (10): 927–938. doi:10.1001/jama.2017.11217. PMID 28898378.
- ↑ Langer, RD; Hodis, HN; Lobo, RA; Allison, MA (February 2021). "Hormone replacement therapy – where are we now?". Climacteric 24 (1): 3–10. doi:10.1080/13697137.2020.1851183. PMID 33403881.
- ↑ 8.0 8.1 8.2 Løkkegaard, E; Nielsen, LH; Keiding, N (August 2017). "Risk of Stroke With Various Types of Menopausal Hormone Therapies: A National Cohort Study.". Stroke 48 (8): 2266–2269. doi:10.1161/STROKEAHA.117.017132. PMID 28626058.
- ↑ 9.0 9.1 9.2 9.3 Files, JA; Ko, MG; Pruthi, S (July 2011). "Bioidentical hormone therapy.". Mayo Clinic Proceedings 86 (7): 673–80, quiz 680. doi:10.4065/mcp.2010.0714. PMID 21531972.
- ↑ "Bioidentical hormones". Cleveland Clinic. 12 December 2012. https://my.clevelandclinic.org/health/articles/15660-bioidentical-hormones.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "Bioidentical hormones: an evidence-based review for primary care providers". J Am Osteopath Assoc 111 (3): 153–64. March 2011. PMID 21464264. http://jaoa.org/article.aspx?articleid=2094168.
- ↑ 12.0 12.1 12.2 Cobin, RH; Goodman, NF; AACE Reproductive Endocrinology Scientific, Committee. (1 July 2017). "Position Statement on Menopause – 2017 Update". Endocrine Practice 23 (7): 869–880. doi:10.4158/EP171828.PS. PMID 28703650. https://www.aace.com/files/position-statements/ep171828ps.pdf. Retrieved 1 March 2019.
- ↑ 13.0 13.1 "USPTF Consensus Statement". 2012. http://www.uspreventiveservicestaskforce.org/uspstf12/menohrt/menohrtart.htm.
- ↑ Lewis, Ricki. "ACOG Revises Guidelines on Treating Menopause Symptoms". Medscape. https://www.medscape.com/viewarticle/818280.
- ↑ "Hormone Therapy and Heart Disease – ACOG". Committee on Gynecologic Practice. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Hormone-Therapy-and-Heart-Disease.
- ↑ "Medscape". http://www.medscape.com/viewarticle/869397.
- ↑ 17.0 17.1 Santen, RJ; Utian, WH (2010). "Executive Summary: Postmenopausal Hormone Therapy: An Endocrine Society Scientific Statement". J Clin Endocrinol Metab 95 S1–S66 (Supplement 1): s1–s66. doi:10.1210/jc.2009-2509. PMID 20566620.
- ↑ 18.0 18.1 18.2 18.3 Marjoribanks, Jane; Farquhar, Cindy; Roberts, Helen; Lethaby, Anne; Lee, Jasmine (17 January 2017). "Long-term hormone therapy for perimenopausal and postmenopausal women". The Cochrane Database of Systematic Reviews 1 (1): CD004143. doi:10.1002/14651858.CD004143.pub5. ISSN 1469-493X. PMID 28093732.
- ↑ "Menopause treatments". National Health Service, United Kingdom. 2019. https://www.nhs.uk/conditions/menopause/treatment/.
- ↑ "Skin aging and menopause: implications for treatment". Am J Clin Dermatol 4 (6): 371–8. 2003. doi:10.2165/00128071-200304060-00001. PMID 12762829.
- ↑ "Hormonal therapy of intrinsic aging". Rejuvenation Res 15 (3): 302–12. June 2012. doi:10.1089/rej.2011.1249. PMID 22533363.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 Sarrel, P.M. (2000). Effects of hormone replacement therapy on sexual psychophysiology and behavior in postmenopause. Journal of Women's Health and Gender-Based Medicine, 9, 25–32
- ↑ 23.0 23.1 23.2 23.3 23.4 Boardman, HM; Hartley, L; Eisinga, A; Main, C; Roqué i Figuls, M; Bonfill Cosp, X; Gabriel Sanchez, R; Knight, B (10 March 2015). "Hormone therapy for preventing cardiovascular disease in post-menopausal women.". The Cochrane Database of Systematic Reviews (3): CD002229. doi:10.1002/14651858.CD002229.pub4. ISSN 1469-493X. PMID 25754617. https://semanticscholar.org/paper/ca6ab03a39fb55f28caf4b2925dda015257e7e66.
- ↑ Hodis, HN; Mack, WJ; Henderson, VW; Shoupe, D; Budoff, MJ; Hwang-Levine, J; Li, Y; Feng, M et al. (31 March 2016). "Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol.". The New England Journal of Medicine 374 (13): 1221–31. doi:10.1056/NEJMoa1505241. PMID 27028912.
- ↑ Writing Group on Behalf of Workshop Consensus (October 2009). "Aging, menopause, cardiovascular disease and HRT. International Menopause Society Consensus Statement.". Climacteric 12 (5): 368–77. doi:10.1080/13697130903195606. PMID 19811229.
- ↑ 26.0 26.1 26.2 26.3 26.4 Manson, JE; Chlebowski, RT; Stefanick, ML; Aragaki, AK; Rossouw, JE; Prentice, RL; Anderson, G; Howard, BV et al. (2 October 2013). "Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials.". JAMA 310 (13): 1353–68. doi:10.1001/jama.2013.278040. PMID 24084921.
- ↑ 27.0 27.1 Saeaib, N; Peeyananjarassri, K; Liabsuetrakul, T; Buhachat, R; Myriokefalitaki, E (28 January 2020). "Hormone replacement therapy after surgery for epithelial ovarian cancer.". The Cochrane Database of Systematic Reviews 1 (1): CD012559. doi:10.1002/14651858.CD012559.pub2. PMID 31989588.
- ↑ Darabi, M.; Rabbani, M.; Ani, M.; Zarean, E.; Panjehpour, M.; Movahedian, A. (2011). "Increased leukocyte ABCA1 gene expression in post-menopausal women on hormone replacement therapy". Gynecological Endocrinology 27 (9): 701–705. doi:10.3109/09513590.2010.507826. PMID 20807164.
- ↑ Gregersen, I; Høibraaten, E; Holven, KB; Løvdahl, L; Ueland, T; Mowinckel, MC; Dahl, TB; Aukrust, P et al. (December 2019). "Effect of hormone replacement therapy on atherogenic lipid profile in postmenopausal women.". Thrombosis Research 184: 1–7. doi:10.1016/j.thromres.2019.10.005. PMID 31677448.
- ↑ Spoletini, I; Vitale, C; Pelliccia, F; Fossati, C; Rosano, GM (December 2014). "Androgens and cardiovascular disease in postmenopausal women: a systematic review.". Climacteric 17 (6): 625–34. doi:10.3109/13697137.2014.887669. PMID 24559253.
- ↑ 31.0 31.1 31.2 31.3 Davis, SR; Baber, R; Panay, N; Bitzer, J; Perez, SC; Islam, RM; Kaunitz, AM; Kingsberg, SA et al. (September 2019). "Global Consensus Position Statement on the Use of Testosterone Therapy for Women.". The Journal of Sexual Medicine 16 (9): 1331–1337. doi:10.1016/j.jsxm.2019.07.012. PMID 31488288. https://www.openaccessrepository.it/record/43710.
- ↑ 32.0 32.1 32.2 Shifren, JL; Davis, SR (August 2017). "Androgens in postmenopausal women: a review.". Menopause 24 (8): 970–979. doi:10.1097/GME.0000000000000903. PMID 28609390.
- ↑ 33.0 33.1 Scarabin, P.-Y. (August 2018). "Progestogens and venous thromboembolism in menopausal women: an updated oral versus transdermal estrogen meta-analysis". Climacteric 21 (4): 341–345. doi:10.1080/13697137.2018.1446931. ISSN 1473-0804. PMID 29570359.
- ↑ Olié, V. R.; Canonico, M.; Scarabin, P. Y. (2010). "Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women". Current Opinion in Hematology 17 (5): 457–463. doi:10.1097/MOH.0b013e32833c07bc. PMID 20601871.
- ↑ Rott, H (2014). "Prevention and treatment of venous thromboembolism during HRT: current perspectives.". International Journal of General Medicine 7: 433–40. doi:10.2147/IJGM.S46310. PMID 25210472.
- ↑ 36.0 36.1 "Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies". PLOS Med. 14 (11): e1002445. November 2017. doi:10.1371/journal.pmed.1002445. PMID 29149179.
- ↑ Chlebowski, RT; Anderson, GL; Sarto, GE; Haque, R; Runowicz, CD; Aragaki, AK; Thomson, CA; Howard, BV et al. (March 2016). "Continuous Combined Estrogen Plus Progestin and Endometrial Cancer: The Women's Health Initiative Randomized Trial.". Journal of the National Cancer Institute 108 (3): djv350. doi:10.1093/jnci/djv350. PMID 26668177.
- ↑ Archer, DF (2001). "The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women.". Menopause 8 (4): 245–51. doi:10.1097/00042192-200107000-00005. PMID 11449081.
- ↑ 39.0 39.1 39.2 Kim, JJ; Kurita, T; Bulun, SE (February 2013). "Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer.". Endocrine Reviews 34 (1): 130–62. doi:10.1210/er.2012-1043. PMID 23303565.
- ↑ Young, Robert; Arlan F., Jr Fuller; Fuller, Arlan F.; Michael V. Seiden (2004). Uterine cancer. Hamilton, Ont: B.C. Decker. ISBN 978-1-55009-163-2.
- ↑ Zang, H; Sahlin, L; Masironi, B; Eriksson, E; Lindén Hirschberg, A (June 2007). "Effects of testosterone treatment on endometrial proliferation in postmenopausal women.". The Journal of Clinical Endocrinology and Metabolism 92 (6): 2169–75. doi:10.1210/jc.2006-2171. PMID 17341565.
- ↑ "Endometrial cancer risk was lower in women who used continuous combined HRT than in non-users". Evidence-based Obstetrics & Gynecology 8 (1–2): 68–69. March 2006. doi:10.1016/j.ebobgyn.2006.01.011. ISSN 1361-259X.
- ↑ 43.0 43.1 43.2 Zeng, Z; Jiang, X; Li, X; Wells, A; Luo, Y; Neapolitan, R (2018). "Conjugated equine estrogen and medroxyprogesterone acetate are associated with decreased risk of breast cancer relative to bioidentical hormone therapy and controls.". PLOS ONE 13 (5): e0197064. doi:10.1371/journal.pone.0197064. PMID 29768475. Bibcode: 2018PLoSO..1397064Z.
- ↑ Fournier, A. S.; Berrino, F.; Clavel-Chapelon, F. O. (2007). "Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study". Breast Cancer Research and Treatment 107 (1): 103–111. doi:10.1007/s10549-007-9523-x. PMID 17333341.
- ↑ Beral, V; Reeves, G; Bull, D; Green, J; Million Women Study, Collaborators. (16 February 2011). "Breast cancer risk in relation to the interval between menopause and starting hormone therapy.". Journal of the National Cancer Institute 103 (4): 296–305. doi:10.1093/jnci/djq527. PMID 21278356.
- ↑ Letendre, I.; Lopes, P. (2012). "Ménopause et risques carcinologiques". Journal de Gynécologie Obstétrique et Biologie de la Reproduction 41 (7): F33–F37. doi:10.1016/j.jgyn.2012.09.006. PMID 23062839.
- ↑ Chlebowski, RT; Anderson, GL; Aragaki, AK; Manson, JE; Stefanick, ML; Pan, K; Barrington, W; Kuller, LH et al. (28 July 2020). "Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women's Health Initiative Randomized Clinical Trials.". JAMA 324 (4): 369–380. doi:10.1001/jama.2020.9482. PMID 32721007.
- ↑ Anderson, G. L.; Limacher, M.; Assaf, A. R.; Bassford, T.; Beresford, S. A.; Black, H.; Bonds, D.; Brunner, R. et al. (2004). "Effects of Conjugated Equine Estrogen in Postmenopausal Women with Hysterectomy: The Women's Health Initiative Randomized Controlled Trial". JAMA: The Journal of the American Medical Association 291 (14): 1701–1712. doi:10.1001/jama.291.14.1701. PMID 15082697.
- ↑ Stefanick ML et al. (2006). "Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy". JAMA 295 (14): 1647–57. doi:10.1001/jama.295.14.1647. PMID 16609086.
- ↑ "Association between hormone replacement therapy use and breast cancer risk varies by race/ethnicity, body mass index, and breast density". JNCI Journal of the National Cancer Institute 105 (18). 2013. doi:10.1093/jnci/djt264. ISSN 0027-8874.
- In turn citing: Hou, N.; Hong, S.; Wang, W.; Olopade, O. I.; Dignam, J. J.; Huo, D. (2013). "Hormone Replacement Therapy and Breast Cancer: Heterogeneous Risks by Race, Weight, and Breast Density". JNCI Journal of the National Cancer Institute 105 (18): 1365–1372. doi:10.1093/jnci/djt207. ISSN 0027-8874. PMID 24003037.
- ↑ Kuhl, H.; Schneider, H. P. G. (2013). "Progesterone – promoter or inhibitor of breast cancer". Climacteric 16 Suppl 1: 54–68. doi:10.3109/13697137.2013.768806. PMID 23336704.
- ↑ Azam, S; Lange, T; Huynh, S; Aro, AR; von Euler-Chelpin, M; Vejborg, I; Tjønneland, A; Lynge, E et al. (June 2018). "Hormone replacement therapy, mammographic density, and breast cancer risk: a cohort study.". Cancer Causes & Control 29 (6): 495–505. doi:10.1007/s10552-018-1033-0. PMID 29671181.
- ↑ Stute, P; Wildt, L; Neulen, J (April 2018). "The impact of micronized progesterone on breast cancer risk: a systematic review.". Climacteric 21 (2): 111–122. doi:10.1080/13697137.2017.1421925. PMID 29384406.
- ↑ 54.0 54.1 Panay, N; Medical Advisory Council of the British Menopause, Society. (June 2019). "BMS – Consensus statement: Bioidentical HRT.". Post Reproductive Health 25 (2): 61–63. doi:10.1177/2053369119841844. PMID 31192760.
- ↑ Management of the menopause after breast cancer , from The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. College Statement C-Gyn 15. 1st Endorsed: February 2003. Current: November 2011. Review: November 2014
- ↑ Gordhandas, S; Norquist, BM; Pennington, KP; Yung, RL; Laya, MB; Swisher, EM (April 2019). "Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits.". Gynecologic Oncology 153 (1): 192–200. doi:10.1016/j.ygyno.2018.12.014. PMID 30661763.
- ↑ Heinig, M; Schwarz, S; Haug, U (16 February 2021). "Self-selection for mammography screening according to use of hormone replacement therapy: A systematic literature review.". Cancer Epidemiology 71 (Pt A): 101812. doi:10.1016/j.canep.2020.101812. PMID 33608235. https://repository.publisso.de/resource/frl:6425860.
- ↑ Kotsopoulos, J; Narod, SA (January 2012). "Androgens and breast cancer.". Steroids 77 (1–2): 1–9. doi:10.1016/j.steroids.2011.10.002. PMID 22015396.
- ↑ Collaborative Group on Epidemiological Studies of Ovarian Cancer (12 February 2015). "Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies". The Lancet 385 (9980): 1835–1842. doi:10.1016/S0140-6736(14)61687-1. PMID 25684585.
- ↑ 60.0 60.1 60.2 Shi, LF; Wu, Y; Li, CY (April 2016). "Hormone therapy and risk of ovarian cancer in postmenopausal women: a systematic review and meta-analysis.". Menopause 23 (4): 417–24. doi:10.1097/GME.0000000000000550. PMID 26506499.
- ↑ Guidozzi, F (December 2013). "Estrogen therapy in gynecological cancer survivors.". Climacteric 16 (6): 611–7. doi:10.3109/13697137.2013.806471. PMID 23952524.
- ↑ "Menopausal Hormone Therapy and Cancer Risk" (in en). www.cancer.org. https://www.cancer.org/cancer/cancer-causes/medical-treatments/menopausal-hormone-replacement-therapy-and-cancer-risk.html.
- ↑ Jang, YC; Huang, HL; Leung, CY (9 December 2019). "Association of hormone replacement therapy with mortality in colorectal cancer survivor: a systematic review and meta-analysis.". BMC Cancer 19 (1): 1199. doi:10.1186/s12885-019-6428-0. PMID 31818262.
- ↑ Vargiu, V; Amar, ID; Rosati, A; Dinoi, G; Turco, LC; Capozzi, VA; Scambia, G; Villa, P (25 November 2020). "Hormone replacement therapy and cervical cancer: a systematic review of the literature.". Climacteric 24 (2): 120–127. doi:10.1080/13697137.2020.1826426. PMID 33236658.
- ↑ Miller M.M.; Franklin K.B.J. (1999). "Theoretical basis for the benefit of postmenopausal estrogen substitution". Experimental Gerontology 34 (5): 587–604. doi:10.1016/S0531-5565(99)00032-7. PMID 10530785.
- ↑ Gonzalez M., Viagara G., Caba F., Molina E. (2004). "Sexual function, menopause and hormone replacement therapy (HRT)". The European Menopause Journal 48 (4): 411–420. doi:10.1016/j.maturitas.2003.10.005. PMID 15283933.
- ↑ Johansen, N; Lindén Hirschberg, A; Moen, MH (August 2020). "The role of testosterone in menopausal hormone treatment. What is the evidence?". Acta Obstetricia et Gynecologica Scandinavica 99 (8): 966–969. doi:10.1111/aogs.13819. PMID 32027015.
- ↑ 68.0 68.1 Dye, RV; Miller, KJ; Singer, EJ; Levine, AJ (2012). "Hormone replacement therapy and risk for neurodegenerative diseases.". International Journal of Alzheimer's Disease 2012: 258454. doi:10.1155/2012/258454. PMID 22548198.
- ↑ Campdelacreu, J (November 2014). "Parkinson disease and Alzheimer disease: environmental risk factors.". Neurologia (Barcelona, Spain) 29 (9): 541–9. doi:10.1016/j.nrl.2012.04.001. PMID 22703631.
- ↑ Nicoletti, A; Nicoletti, G; Arabia, G; Annesi, G; De Mari, M; Lamberti, P; Grasso, L; Marconi, R et al. (December 2011). "Reproductive factors and Parkinson's disease: a multicenter case-control study.". Movement Disorders 26 (14): 2563–6. doi:10.1002/mds.23951. PMID 21956541.
- ↑ Noyce, AJ; Bestwick, JP; Silveira-Moriyama, L; Hawkes, CH; Giovannoni, G; Lees, AJ; Schrag, A (December 2012). "Meta-analysis of early nonmotor features and risk factors for Parkinson disease.". Annals of Neurology 72 (6): 893–901. doi:10.1002/ana.23687. PMID 23071076.
- ↑ Wang, P; Li, J; Qiu, S; Wen, H; Du, J (2015). "Hormone replacement therapy and Parkinson's disease risk in women: a meta-analysis of 14 observational studies.". Neuropsychiatric Disease and Treatment 11: 59–66. doi:10.2147/NDT.S69918. PMID 25657580.
- ↑ Rugbjerg, K; Christensen, J; Tjønneland, A; Olsen, JH (April 2013). "Exposure to estrogen and women's risk for Parkinson's disease: a prospective cohort study in Denmark.". Parkinsonism & Related Disorders 19 (4): 457–60. doi:10.1016/j.parkreldis.2013.01.008. PMID 23402992.
- ↑ Yoon, BK; Chin, J; Kim, JW; Shin, MH; Ahn, S; Lee, DY; Seo, SW; Na, DL (August 2018). "Menopausal hormone therapy and mild cognitive impairment: a randomized, placebo-controlled trial.". Menopause 25 (8): 870–876. doi:10.1097/GME.0000000000001140. PMID 29846283.
- ↑ Rasgon, NL; Geist, CL; Kenna, HA; Wroolie, TE; Williams, KE; Silverman, DH (2014). "Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia.". PLOS ONE 9 (3): e89095. doi:10.1371/journal.pone.0089095. PMID 24622517. Bibcode: 2014PLoSO...989095R.
- ↑ "Estrogen replacement therapy in older women: a neuropsychological and brain MRI study". Journal of the American Geriatrics Society 44 (11): 1307–13. November 1996. doi:10.1111/j.1532-5415.1996.tb01400.x. PMID 8909345.
- ↑ Parkinson Study Group POETRY, Investigators. (December 2011). "A randomized pilot trial of estrogen replacement therapy in post-menopausal women with Parkinson's disease.". Parkinsonism & Related Disorders 17 (10): 757–60. doi:10.1016/j.parkreldis.2011.07.007. PMID 21824799.
- ↑ Diamanti-Kandarakis, E; Dattilo, M; Macut, D; Duntas, L; Gonos, ES; Goulis, DG; Gantenbein, CK; Kapetanou, M et al. (June 2017). "Mechanisms in Endocrinology: Aging and anti-aging: a Combo-Endocrinology overview.". European Journal of Endocrinology 176 (6): R283–R308. doi:10.1530/EJE-16-1061. PMID 28264815.
- ↑ Vest, RS; Pike, CJ (February 2013). "Gender, sex steroid hormones, and Alzheimer's disease.". Hormones and Behavior 63 (2): 301–7. doi:10.1016/j.yhbeh.2012.04.006. PMID 22554955.
- ↑ Pike, CJ; Carroll, JC; Rosario, ER; Barron, AM (July 2009). "Protective actions of sex steroid hormones in Alzheimer's disease.". Frontiers in Neuroendocrinology 30 (2): 239–58. doi:10.1016/j.yfrne.2009.04.015. PMID 19427328.
- ↑ Zhu, L; Jiang, X; Sun, Y; Shu, W (April 2016). "Effect of hormone therapy on the risk of bone fractures: a systematic review and meta-analysis of randomized controlled trials.". Menopause 23 (4): 461–70. doi:10.1097/GME.0000000000000519. PMID 26529613.
- ↑ 82.0 82.1 Studd J (March 2010). "Ten reasons to be happy about hormone replacement therapy: a guide for patients". Menopause Int. 16 (1): 44–6. doi:10.1258/mi.2010.010001. PMID 20424287. https://semanticscholar.org/paper/35f42df732e8b9041df4756a78b337b58adf6cdb.
- ↑ Tiidus, PM (August 2011). "Benefits of estrogen replacement for skeletal muscle mass and function in post-menopausal females: evidence from human and animal studies.". The Eurasian Journal of Medicine 43 (2): 109–14. doi:10.5152/eajm.2011.24. PMID 25610174.
- ↑ Deleruyelle, LJ (2017). "Menopausal Symptom Relief and Side Effects Experienced by Women Using Compounded Bioidentical Hormone Replacement Therapy and Synthetic Conjugated Equine Estrogen and/or Progestin Hormone Replacement Therapy, Part 3.". International Journal of Pharmaceutical Compounding 21 (1): 6–16. PMID 28346192.
- ↑ "A case of late-onset chorea". Nat Clin Pract Neurol 1 (2): 113–6. December 2005. doi:10.1038/ncpneuro0052. PMID 16932507. http://www.nature.com/ncpneuro/journal/v1/n2/full/ncpneuro0052.html.
- ↑ MacLennan, AH (August 2011). "HRT in difficult circumstances: are there any absolute contraindications?". Climacteric 14 (4): 409–17. doi:10.3109/13697137.2010.543496. PMID 21355685.
- ↑ 87.0 87.1 87.2 87.3 87.4 87.5 87.6 87.7 87.8 87.9 Fugh-Berman, Adriane (2015). "The Science of Marketing: How Pharmaceutical Companies Manipulated Medical Discourse on Menopause". Women's Reproductive Health 2 (1): 18–23. doi:10.1080/23293691.2015.1039448. ISSN 2329-3691.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 205–. ISBN 978-92-832-1291-1. https://books.google.com/books?id=aGDU5xibtNgC&pg=PA205.
- ↑ 89.0 89.1 89.2 Writing Group for the Women's Health Initiative Investigators (2002). "Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women's Health Initiative Randomized Controlled Trial". JAMA 288 (3): 321–333. doi:10.1001/jama.288.3.321. PMID 12117397.
- ↑ "Breast cancer after use of estrogen plus progestin in postmenopausal women". The New England Journal of Medicine 360 (6): 573–87. February 2009. doi:10.1056/NEJMoa0807684. PMID 19196674.
- ↑ Kreatsoulas, C.; Anand, S. S. (2013). "Menopausal hormone therapy for the primary prevention of chronic conditions. U.S. Preventive Services Task Force Recommendation Statement". Polskie Archiwum Medycyny Wewnetrznej 123 (3): 112–117. PMID 23396275. http://pamw.pl/sites/default/files/PAMW_2013-3_Anand.pdf.
- ↑ Nelson, H. D.; Walker, M.; Zakher, B.; Mitchell, J. (2012). "Menopausal hormone therapy for the primary prevention of chronic conditions: A systematic review to update the U.S. Preventive Services Task Force recommendations". Annals of Internal Medicine 157 (2): 104–113. doi:10.7326/0003-4819-157-2-201207170-00466. PMID 22786830.
- ↑ George, James L.; Colman, Robert W.; Goldhaber, Samuel Z.; Victor J. Marder (2006). Hemostasis and thrombosis: basic principles and clinical practice. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 1239. ISBN 978-0-7817-4996-1.
- ↑ "Effect of estrogen receptor β A1730G polymorphism on ABCA1 gene expression response to postmenopausal hormone replacement therapy". Genetic Testing and Molecular Biomarkers 15 (1–2): 11–5. 2011. doi:10.1089/gtmb.2010.0106. PMID 21117950.
- ↑ Chlebowski, R. T.; Anderson, G. L. (2015). "Menopausal hormone therapy and breast cancer mortality: clinical implications". Therapeutic Advances in Drug Safety 6 (2): 45–56. doi:10.1177/2042098614568300. ISSN 2042-0986. PMID 25922653.
- ↑ "Hormone Therapy and Menopause". December 2010. http://www.center4research.org/hormone-therapy-menopause/.
- ↑ Gina Kolata (19 April 2007). "Sharp Drop in Rates of Breast Cancer Holds". New York Times. https://query.nytimes.com/gst/fullpage.html?res=9A03E6D91E3FF93AA25757C0A9619C8B63.
- ↑ Roni Caryn Rabin (28 August 2007). "For a Low-Dose Hormone, Take Your Pick". New York Times. https://query.nytimes.com/gst/fullpage.html?res=9D03E6D91539F93BA1575BC0A9619C8B63&sec=&spon=. "Many women seeking natural remedies have turned to compounding pharmacies, which use bioidentical hormones that are chemically synthesized but with the same molecular structure as hormones produced by a woman's body."
- ↑ John Gever (5 April 2011). "New WHI Estrogen Analysis Shows Lower Breast Ca Risk". MedPageToday. http://www.medpagetoday.com/OBGYN/HRT/25734.
- ↑ Salpeter, S. R.; Cheng, J.; Thabane, L.; Buckley, N. S.; Salpeter, E. E. (2009). "Bayesian Meta-analysis of Hormone Therapy and Mortality in Younger Postmenopausal Women". The American Journal of Medicine 122 (11): 1016–1022.e1. doi:10.1016/j.amjmed.2009.05.021. PMID 19854329.
- ↑ Anderson, G. L.; Chlebowski, R. T.; Rossouw, J. E.; Rodabough, R. J.; McTiernan, A.; Margolis, K. L.; Aggerwal, A.; David Curb, J. D. et al. (2006). "Prior hormone therapy and breast cancer risk in the Women's Health Initiative randomized trial of estrogen plus progestin". Maturitas 55 (2): 103–115. doi:10.1016/j.maturitas.2006.05.004. PMID 16815651.
- ↑ Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. (1998). "Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group". JAMA: The Journal of the American Medical Association 280 (7): 605–613. doi:10.1001/jama.280.7.605. PMID 9718051.
- ↑ Salpeter, S. R.; Walsh, J. M. E.; Greyber, E.; Ormiston, T. M.; Salpeter, E. E. (2004). "Mortality associated with hormone replacement therapy in younger and older women". Journal of General Internal Medicine 19 (7): 791–804. doi:10.1111/j.1525-1497.2004.30281.x. PMID 15209595.
- ↑ Grodstein, F.; Stampfer, M. J.; Colditz, G. A.; Willett, W. C.; Manson, J. E.; Joffe, M.; Rosner, B.; Fuchs, C. et al. (1997). "Postmenopausal Hormone Therapy and Mortality". New England Journal of Medicine 336 (25): 1769–1775. doi:10.1056/NEJM199706193362501. PMID 9187066.
- ↑ Bethea CL (Feb 2011). "MPA: Medroxy-Progesterone Acetate Contributes to Much Poor Advice for Women". Endocrinology 152 (2): 343–345. doi:10.1210/en.2010-1376. PMID 21252179.
- ↑ "KEEPS: The Kronos Early Estrogen Prevention Study". Climacteric 8 (1): 3–12. March 2005. doi:10.1080/13697130500042417. PMID 15804727.
- ↑ Dominus, Susan (2023-02-01). "Women Have Been Misled About Menopause" (in en-US). The New York Times. ISSN 0362-4331. https://www.nytimes.com/2023/02/01/magazine/menopause-hot-flashes-hormone-therapy.html. "No research has yet followed women who start in their 50s and stay on continuously into their 60s."
- ↑ "Androgens and women at the menopause and beyond". J. Gerontol. A Biol. Sci. Med. Sci. 58 (5): M409–16. May 2003. doi:10.1093/gerona/58.5.M409. PMID 12730248.
- ↑ "Role of androgens, progestins and tibolone in the treatment of menopausal symptoms: a review of the clinical evidence". Clin Interv Aging 3 (1): 1–8. 2008. doi:10.2147/CIA.S1043. PMID 18488873.
- ↑ Formoso, G; Perrone, E; Maltoni, S; Balduzzi, S; Wilkinson, J; Basevi, V; Marata, AM; Magrini, N et al. (12 October 2016). "Short-term and long-term effects of tibolone in postmenopausal women.". The Cochrane Database of Systematic Reviews 10 (10): CD008536. doi:10.1002/14651858.CD008536.pub3. ISSN 1469-493X. PMID 27733017.
- ↑ Cummings, SR; Ettinger, B; Delmas, PD; Kenemans, P; Stathopoulos, V; Verweij, P; Mol-Arts, M; Kloosterboer, L et al. (14 August 2008). "The effects of tibolone in older postmenopausal women.". The New England Journal of Medicine 359 (7): 697–708. doi:10.1056/NEJMoa0800743. PMID 18703472.
- ↑ Estrogen (Vaginal Route) from Mayo Clinic / Thomson Healthcare Inc. Portions of this document last updated: 1 November 2011
- ↑ Somboonporn, W; Davis, S; Seif, MW; Bell, R (19 October 2005). "Testosterone for peri- and postmenopausal women.". The Cochrane Database of Systematic Reviews (4): CD004509. doi:10.1002/14651858.CD004509.pub2. PMID 16235365. https://semanticscholar.org/paper/61d86a4f71f51c54e66a8621da7a5c3aed31bc9f.
- ↑ North American Menopause, Society. (2005). "The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society.". Menopause 12 (5): 496–511; quiz 649. doi:10.1097/01.gme.0000177709.65944.b0. PMID 16145303. https://semanticscholar.org/paper/5a4d908cac38d7b4c3fe66023314c1e0ee913d8e.
- ↑ Drapier-Faure, E; Azoulay, C; Abramovici, Y (July 2005). "[Acceptability of continuous combined versus cyclical HRT: a French multicentric randomized clinical study].". Gynécologie, obstétrique & fertilité 33 (7–8): 498–504. doi:10.1016/j.gyobfe.2005.04.024. PMID 16005674.
- ↑ Feeley, KM; Wells, M (June 2001). "Hormone replacement therapy and the endometrium.". Journal of Clinical Pathology 54 (6): 435–40. doi:10.1136/jcp.54.6.435. PMID 11376015.
- ↑ Junkermann, H; von Holst, T; Lang, E; Rakov, V (14 February 2005). "Influence of different HRT regimens on mammographic density.". Maturitas 50 (2): 105–10. doi:10.1016/j.maturitas.2004.04.008. PMID 15653007.
- ↑ 118.0 118.1 Beck, KL; Anderson, MC; Kirk, JK (August 2017). "Transdermal estrogens in the changing landscape of hormone replacement therapy.". Postgraduate Medicine 129 (6): 632–636. doi:10.1080/00325481.2017.1334507. PMID 28540770.
- ↑ 119.0 119.1 119.2 119.3 119.4 119.5 119.6 "Drug delivery systems for hormone therapy". J Control Release 112 (1): 1–14. May 2006. doi:10.1016/j.jconrel.2006.01.021. PMID 16530874.
- ↑ 120.0 120.1 120.2 120.3 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 Suppl 1: 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ Martin, K. A.; Barbieri, R. L.; Crowley, W. F. Jr. (24 September 2021). "Preparations for menopausal hormone therapy". UpToDate. https://www.uptodate.com/contents/preparations-for-menopausal-hormone-therapy. Retrieved 2 February 2022. "Not recommended. Depot estrogen—Estrogen is also available in some, but not all, countries as long-acting (three to four weeks) injections of either estradiol cypionate or estradiol valerate. Given the other effective estrogen options, we do not use this approach (table 1)."
- ↑ "Different routes of progesterone administration and polycystic ovary syndrome: a review of the literature". Gynecol Endocrinol 21 (2): 119–27. August 2005. doi:10.1080/09513590500170049. PMID 16109599.
- ↑ 123.0 123.1 "FDA Takes Action Against Compounded Menopause Hormone Therapy Drugs". FDA. 9 January 2008. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116832.htm.
- ↑ Cirigliano, M (June 2007). "Bioidentical hormone therapy: a review of the evidence.". Journal of Women's Health 16 (5): 600–31. doi:10.1089/jwh.2006.0311. PMID 17627398.
- ↑ "What if the Women's Health Initiative had used transdermal estradiol and oral progesterone instead?". Menopause 21 (7): 769–83. July 2014. doi:10.1097/GME.0000000000000169. PMID 24398406.
- ↑ 126.0 126.1 Pinkerton, JV; Pickar, JH (February 2016). "Update on medical and regulatory issues pertaining to compounded and FDA-approved drugs, including hormone therapy.". Menopause 23 (2): 215–23. doi:10.1097/GME.0000000000000523. PMID 26418479.
- ↑ 127.0 127.1 127.2 127.3 Singer, Natasha (4 August 2009). "Medical Papers by Ghostwriters Pushed Therapy". The New York Times. https://www.nytimes.com/2009/08/05/health/research/05ghost.html.
- ↑ 128.0 128.1 128.2 128.3 128.4 128.5 "The haunting of medical journals: how ghostwriting sold "HRT"". PLOS Med. 7 (9): e1000335. September 2010. doi:10.1371/journal.pmed.1000335. PMID 20838656.
- ↑ 129.0 129.1 129.2 129.3 129.4 129.5 129.6 Steve Kent May; Steve May (20 January 2012). Case Studies in Organizational Communication: Ethical Perspectives and Practices: Ethical Perspectives and Practices. SAGE. pp. 197–. ISBN 978-1-4129-8309-9. https://books.google.com/books?id=g7hduew1u1IC&pg=PA197.
- ↑ 130.0 130.1 "An update on hormone therapy in postmenopausal women: mini-review for the basic scientist". Am. J. Physiol. Heart Circ. Physiol. 313 (5): H1013–H1021. November 2017. doi:10.1152/ajpheart.00383.2017. PMID 28801526.
- ↑ Sorkin, Andrew Ross; Wilson, Duff (25 January 2009). "Pfizer Agrees to Pay $68 Billion for Rival Drug Maker Wyeth". The New York Times. https://www.nytimes.com/2009/01/26/business/26drug.html.
- ↑ "Pfizer Sizes Up: Closes $68B Wyeth Deal". 19 October 2009. https://www.cnbc.com/id/33384753.
- ↑ "Knowledge, perceptions and information about hormone therapy (HT) among menopausal women: a systematic review and meta-synthesis". PLOS ONE 6 (9): e24661. 2011. doi:10.1371/journal.pone.0024661. PMID 21949743. Bibcode: 2011PLoSO...624661T.
- ↑ "Hormone therapy for brain performance: No effect, whether started early or late". https://www.sciencedaily.com/releases/2016/07/160720215141.htm.
- ↑ Davies, M; Hamoda, H; British Menopause Society Medical Advisory, Council. (11 February 2019). "Hormone replacement therapy: changes in prescribing practice.". BMJ (Clinical Research Ed.) 364: l633. doi:10.1136/bmj.l633. PMID 30745316.
External links
- Menopause treatment, Hormone Health Network, The Endocrine Society
- Sexual Health and Menopause Online, The North American Menopause Society
- Menopause, US Food and Drug Administration
- British Menopause Society
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
pl:Hormonalna terapia zastępcza
 |
 KSF
KSF