Lymphatic filariasis
Topic: Medicine
 From HandWiki - Reading time: 15 min
From HandWiki - Reading time: 15 min
| Lymphatic filariasis | |
|---|---|
| Other names | Elephantiasis tropica,[1] elephantiasis arabum[1] |
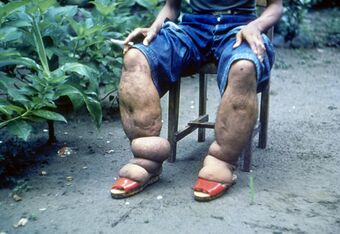 | |
| Elephantiasis of the legs due to filariasis. | |
| Symptoms | None, severe swelling of the arms, legs, breasts, or genitals[2] |
| Causes | Filarial worms spread by mosquitos[3] |
| Diagnostic method | Microscopic examination of blood[4] |
| Prevention | Bed nets, mass deworming[2] |
| Medication | Albendazole with ivermectin or diethylcarbamazine[2] |
| Frequency | 40 million (2022)[5] |
Lymphatic filariasis is a human disease caused by parasitic worms known as filarial worms.[2][3] Usually acquired in childhood, it is a leading cause of permanent disability worldwide, impacting over a hundred million people and manifesting itself in a variety of severe clinical pathologies[6][7] While most cases have no symptoms, some people develop a syndrome called elephantiasis, which is marked by severe swelling in the arms, legs, breasts, or genitals. The skin may become thicker as well, and the condition may become painful.[2] Affected people are often unable to work and are often shunned or rejected by others because of their disfigurement and disability.[7]
It is the first of the mosquito-borne diseases to have been identified.[8] The worms are spread by the bites of infected mosquitoes.[2] Three types of worms are known to cause the disease: Wuchereria bancrofti, Brugia malayi, and Brugia timori, with Wuchereria bancrofti being the most common.[2] These worms damage the lymphatic system by nesting within the lymphatic vessels and disrupting the system's normal function. Worms can survive within the human body for up to 8 years, all while reproducing millions of larvae which circulate through the blood.[9] The disease is diagnosed by microscopic examination of blood collected during the night. The blood is typically examined as a smear after being stained with Giemsa stain. Testing the blood for antibodies against the disease may also permit diagnosis.[4] Other roundworms from the same family are responsible for river blindness.[10]
Prevention can be achieved by treating entire groups in which the disease exists, known as mass deworming.[2] This is done every year for about six years, in an effort to rid a population of the disease entirely.[2] Medications usually include a combination of 2 or more anthelmintic agents: albendazole, ivermectin, and diethylcarbamazine.[11] Efforts to prevent mosquito bites are also recommended, including reducing the number of mosquitoes and promoting the use of bed nets.[2]
As of 2022, about 40 million people were infected, and about 863 million people were at risk of the disease in 47 countries.[5] It is most common in tropical Africa and Asia.[2] Lymphatic filariasis is classified as a neglected tropical disease and one of the four main worm infections.[10] The impact of the disease results in economic losses of billions of dollars a year.[2]
Signs and symptoms
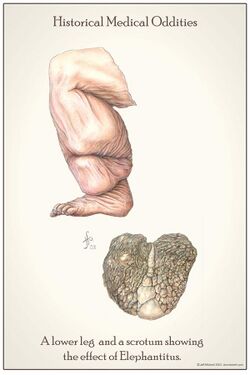
People affected by lymphatic filariasis often experience adverse immunological reactions to the microfilariae as well as the adult worms.[12]: 665 Filariasis may also be associated with ascites following the severe inflammatory reaction in the lymphatics.[13]: 818
Elephantiasis (or "elephantiasis tropica"),[14]: 438 is a dramatic sign of the advanced stage of lymphatic filariasis. Elephantiasis is an advanced stage of lymphedema, characterized by thickening of the skin and underlying tissues of the lower half of the body, causing it to look like that of an elephant. It occurs as a result of the adult worms lodging in the lymphatic system and obstructing the flow of lymph. Different species of filarial worms tend to affect different parts of the body: Wuchereria bancrofti can affect the arms, breasts, legs, scrotum, and vulva (causing hydrocele formation), while Brugia timori rarely affects the genitals.[citation needed]
Causes
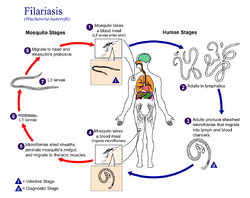
Three species of filarial roundworms, all from the Onchocercidae family, cause human lymphatic filariasis: Wuchereria bancrofti (the most common causative species), Brugia malayi, and Brugia timori. The filarial roundworms are transmitted by the bite of an infected mosquito of genera Aedes, Anopheles, Culex, or Mansonia. Several mosquito bites over several months or years are required in order to develop Lymphatic Filariasis.[15] The mosquito deposits the L3 (infective stage) larvae onto the skin of the human host, where they penetrate into the bite wound. From there, the larvae enter the lymphatic vessels and develop into adults.[7] The larvae take up residence in the lymphatic vessels and the lung tissue, hindering respiration and causing chest pain as the disease progresses.[16] This disease can be confused with tuberculosis,[17] asthma, or coughs related to roundworms.[18]
The disease itself is a result of a complex interplay between several factors: the worm, the endosymbiotic Wolbachia bacteria within the worm, the host's immune response, and the numerous opportunistic infections and disorders that arise. The adult worms live in the human lymphatic system and obstruct the flow of lymph throughout the body; this results in chronic lymphedema, most often noted in the lower torso (typically in the legs and genitals).[19] These worms can survive within the human body for up to 8 years, all while reproducing millions of larvae which circulate through the blood.[9]
Diagnosis
The preferred method for diagnosing lymphatic filariasis is by finding the microfilariae via microscopic examination of the blood. The blood sample is typically in the form of a thick smear, stained with Giemsa stain. Technicians analyzing the blood smear must be able to distinguish between W. bancrofti and other parasites potentially present. A blood smear is a simple and fairly accurate diagnostic tool, provided the blood sample is taken during when the microfilariae are in the peripheral circulation. Because the microfilariae only circulate in the blood at night, the blood specimen must be collected at night.[20]
It is often difficult or impossible to detect the causative organism in the peripheral blood, even in advanced cases.[12] In such cases, testing the blood serum for antibodies against the disease may also be used.[4] A polymerase chain reaction test can also be performed to detect a minute fraction, as little as 1 pg, of filarial DNA.[21] Dead, calcified worms can be detected by X-ray examinations. Ultrasonography can also be used to detect the movements and noises caused by the movement of adult worms.[22]
Differential diagnosis
Lymphatic filariasis may be confused with podoconiosis (also known as nonfilarial elephantiasis), a non-infectious disease caused by exposure of bare feet to irritant alkaline clay soils.[23][24] Podoconiosis however typically affects the legs bilaterally, while filariasis is generally unilateral.[23] Also, podoconiosis very rarely affects the groin while filariasis frequently involves the groin. Geographical location may also help to distinguish between these two diseases: podoconiosis is typically found in higher altitude areas with high seasonal rainfall, while filariasis is common in low-lying areas where mosquitos are prevalent.[23]
Prevention
Protecting against mosquito bites in endemic regions is crucial to the prevention of lymphatic filariasis. Insect repellents and mosquito nets (especially when treated with an insecticide such as deltamethrin or permethrin)[25] have been demonstrated to reduce the transmission of lymphatic filariasis.[26][27] In addition residual spraying and personal protective equipment are known ways to control vectors.[9]
Worldwide eradication of lymphatic filariasis is the definitive goal. This is considered to be achievable since the disease has no known animal reservoir.[26] The World Health Organization (WHO) is coordinating the global effort to eradicate filariasis. The mainstay of this program is mass deworming of entire populations of people who are at risk with antifilarial drugs. The specific treatment depends on the co-endemicity of lymphatic filariasis with other filarial diseases. The WHO's annual MDA guidelines are listed below.
- For areas co-endemic with loiasis 400 mg of albendazole should be administered
- for countries co-endemic with onchocerciasis, 200 mcg/kg of Ivermectin should be administered with 400 mg of albendazole
- in countries without onchocerciasis 6 mg/kg of diethylcarbamazine citrate (DEC) and 400 mg of albendazole should be used
- in countries without onchocerciasis and the IDA Guidelines are met 200 mcg/kg of ivermectin should be used with 6 mg/kg of diethylcarbamazine and 400 mg of albendazole.[9][11]
Because the parasite requires a human host to reproduce, consistent treatment of at-risk populations (annually for a duration of four to six years)[2] is expected to break the cycle of transmission and cause the extinction of the causative organisms.[26]
In 2011, Sri Lanka was certified by the WHO as having eradicated lymphatic filariasis. In July 2017, the WHO announced that the disease had been eliminated in Tonga. Elimination of the disease has also occurred in Cambodia, China, Cook Islands, Egypt, Kiribati, Maldives, Marshall Islands, Niue, Palau, South Korea, Thailand, Vanuatu, Vietnam and Wallis and Futuna.[28] In 2020, the WHO announced that the 2030 targets for this program are that lymphatic filariasis will have been eliminated in 80% of endemic countries.[29]
A vaccine is not yet available, but in 2013, the University of Illinois College of Medicine was reporting 95% efficacy in testing against B. malayi in mice.[30]
Treatment
Treatment of lymphatic filariasis depends in part on the geographic location of the area of the world in which the disease was acquired, but almost always involves the combination of 2 or more anthelmintic agents: albendazole, ivermectin, and diethylcarbamazine. In sub-Saharan Africa, the disease is usually treated with albendazole and ivermectin, whereas in the western pacific region of the world, all 3 anthelmintic agents are used. While Diethylcarbamazine in combination with albendazole is often used, it isn't as region specific as the other combinations.[11]
Wolbachia are endosymbiotic bacteria that live inside the gut of the parasites responsible for lymphatic filariasis, and provide nutrients necessary for their survival. Doxycycline kills these bacteria, which in turn prevents the maturation of microfilariae into adults. It also shortens the lifespan of the adult worms, causing them to die within 1 to 2 years instead of their normal 10 to 14-year lifespan.[31] Doxycycline is effective in treating lymphatic filariasis. Limitations of this antibiotic protocol include that it requires 4 to 6 weeks of treatment rather than the single dose of the anthelmintic agents, that doxycycline should not be used in young children and pregnant women, and that it is phototoxic.[32]
Albendazole is classified as an antihelmintics, which specifically works to kill worms.[33] The drug stops the worms from absorbing glucose, evidently leading to starvation and death from fatigue. The effects of albendazole alone have varying results, however, in combination with DEC drugs it has been found more effective.[34] Ivermectin is administered with albendazole, and works by binding to the nerve cells of the parasites, subsequently making them permeable to chloride. This leads to death by paralysis. Ivermectin, however, has been found to only kill the parasites in their early stages of life, and cannot kill an adult, live worm. Therefore, this drug is usually combined with DEC to kill both the microfilariae and the adult worms.[35]
Surgical treatment may be helpful in cases of scrotal elephantiasis and hydrocele. However, surgery is generally ineffective at correcting elephantiasis of the limbs.[36] Acute inflammatory responses due to lymphedema, and hydrocele can be reduced or prevented by practicing good hygiene, skin care, exercise and elevation of infected limbs.[9]
Epidemiology

Lymphatic filariasis occurs in tropical and subtropical regions of Africa, Asia, Central America, the Caribbean, and South America, and certain Pacific Island nations. Elephantiasis caused by lymphatic filariasis is one of the most common causes of permanent disability in the world.[7] As of 2018, 51 million people were infected with lymphatic filariasis and at least 863 million people in 50 countries were living in areas that require preventive chemotherapy to stop the spread of infection. By 2022, the prevalence had declined to somewhere around 40 million and the disease remains endemic in 47 countries. These improvements are a direct result of the WHO's Global Programme to Eliminate Lymphatic Filariasis.[5] Since implementation, 740 million people no longer require preventative chemotherapy to treat the disease.[9]
W. bancrofti is responsible for 90% of lymphatic filariasis. Brugia malayi causes most of the remainder of the cases, while Brugia timori is a rare cause.[5] W. bancrofti largely affects areas across the broad equatorial belt (Africa, the Nile Delta, Turkey, India, the East Indies, Southeast Asia, Philippines, Oceanic Islands, and parts of South America). Due to the fact that Lymphatic Filariasis requires multiple mosquito bites over several months to years to spread of infection due to tourism is low.[37] The mosquito vectors of W. bancrofti have a preference for human blood; humans are apparently the only animals naturally infected with W. bancrofti. No reservoir host is known.[38] Lymphatic Filariasis is extremely uncommon in the United States, with only one reported case found in South Carolina in the early 1900s.[7]
In South America, four endemic countries have been working to beset lymphatic filariasis, consisting of Brazil, the Dominican Republic, Guyana, and Haiti.[39] In Latin America, the spread of lymphatic filariasis is through W. brancrofti, the only anthropods within the region, culex quinquefasciatus. [40] The exponential rate of development within the Americas is being combated through the development of an MDA program. MDA program, a 3-step drug administering program, has led to a 67% decrease in the need for the drug program.[39] Brazil targeted the rising endemic by administering DEC drugs through an MDA program to the communities hit hardest by the disease. By providing these drugs annually, as well as offering post-care, through showing family members how to treat the disease, creating connections for jobs, as well as providing a social network to incorporate patients into society, Brazil has made the most effort to provide care.[41] Dominican Republic has administered 5 rounds of DEC drugs annually for five years, spanning from 2002-2007. After the initial drastic action, the Dominican then administered another three rounds of MDA. Guyana also used DEC drugs to focus on preventing the spread of the disease, using a DEC-fortified salt from 2003-2007 and ultimately switching to MDA with DEC from 2014 to the present. Targeting patient education and access to treatment.[42] Haiti then focused on the disease by implementing DEC drug in 2002. It reached full geographical coverage by 2012, subsequently in 2014 about 20 communities had eradicated the need for MDA.
In areas endemic for podoconiosis, prevalence can be 5% or higher.[43] In communities where lymphatic filariasis is endemic, as many as 10% of women can be affected by swollen limbs, and 50% of men can develop mutilating genital symptoms.[44]
History

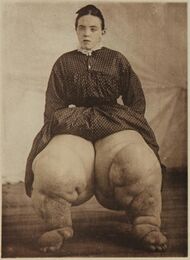
There is evidence of Lymphatic filariasis cases dating back 4000 years.[45] The ancient Vedic text, the Rig Veda, composed around 1500 BC–1200 BC, makes a possible reference to elephantiasis. The 50th hymn of the 7th book of the Rigveda calls on the gods Mitra, Varuna and Agni for protection against "that which nests inside and swells". The author of the hymn implores the deities to not let the worm wound his foot. The disease is described as causing eruptions to appear on the ankles and the knees.[46] Artifacts from ancient Egypt (2000 BC) and the Nok civilization in West Africa (500 BC) show possible elephantiasis symptoms. The first clear reference to the disease occurs in ancient Greek literature, wherein scholars differentiated the often similar symptoms of lymphatic filariasis from those of leprosy, describing leprosy as elephantiasis graecorum and lymphatic filariasis as elephantiasis arabum.[45]
The first documentation of symptoms occurred in the 16th century, when Jan Huyghen van Linschoten wrote about the disease during the exploration of Goa. Similar symptoms were reported by subsequent explorers in areas of Asia and Africa, though an understanding of the disease did not begin to develop until centuries later.[citation needed]
The causative agents were first identified in the late 19th century.[47] In 1866, Timothy Lewis, building on the work of Jean Nicolas Demarquay and Otto Henry Wucherer, made the connection between microfilariae and elephantiasis, establishing the course of research that would ultimately explain the disease. In 1876, Joseph Bancroft discovered the adult form of the worm.[48] In 1877, the lifecycle involving an arthropod vector was theorized by Patrick Manson, who proceeded to demonstrate the presence of the worms in mosquitoes. Manson incorrectly hypothesized that the disease was transmitted through skin contact with water in which the mosquitoes had laid eggs.[49] In 1900, George Carmichael Low determined the actual transmission method by discovering the presence of the worm in the proboscis of the mosquito vector.[45]
| “ | Many people in Malabar, Nayars as well as Brahmans and their wives – in fact about a quarter or a fifth of the total population, including the people of the lowest castes – have very large legs, swollen to a great size; and they die of this, and it is an ugly thing to see. They say that this is due to the water through which they go, because the country is marshy. This is called pericaes in the native language, and all the swelling is the same from the knees downward, and they have no pain, nor do they take any notice of this infirmity. | ” |
| — Portuguese diplomat Tomé Pires, Suma Oriental, 1512–1515.[50] | ||
Research directions
Researchers at the University of Illinois at Chicago (UIC) have developed a novel vaccine for the prevention of lymphatic filariasis. This vaccine has been shown to elicit strong, protective immune responses in mouse models of lymphatic filariasis infection. The immune response elicited by this vaccine has been demonstrated to be protective against both W. bancrofti and B. malayi infection in the mouse model and may prove useful in the human.[51]
On 20 September 2007, geneticists published the first draft of the complete genome (genetic content) of Brugia malayi, one of the roundworms which causes lymphatic filariasis.[52] This project had been started in 1994 and by 2000, 80% of the genome had been determined. Determining the content of the genes might lead to the development of new drugs and vaccines.[53]
Veterinary disease
Onchocerca ochengi causes lymphatic filariasis in cattle.[54][55]
References
- ↑ 1.0 1.1 James, William D.; Berger, Timothy; Elston, Dirk (2015) (in en). Andrews' Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences. p. 432. ISBN 9780323319690. https://books.google.com/books?id=Np6cCQAAQBAJ&pg=PA432.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 "Lymphatic filariasis Fact sheet N°102". World Health Organization. March 2014. https://www.who.int/mediacentre/factsheets/fs102/en/.
- ↑ 3.0 3.1 "Lymphatic filariasis". https://www.who.int/mediacentre/factsheets/fs102/en/.
- ↑ 4.0 4.1 4.2 "Parasites – Lymphatic Filariasis Diagnosis". CDC. 14 June 2013. https://www.cdc.gov/parasites/lymphaticfilariasis/diagnosis.html.
- ↑ 5.0 5.1 5.2 5.3 "Lymphatic filariasis". https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis.
- ↑ Chakraborty, Sanjukta; Gurusamy, Manokaran; Zawieja, David C.; Muthuchamy, Mariappan (July 2013). "Lymphatic filariasis: Perspectives on lymphatic remodeling and contractile dysfunction in filarial disease pathogenesis". Microcirculation 20 (5): 349–364. doi:10.1111/micc.12031. ISSN 1073-9688. PMID 23237232.
- ↑ 7.0 7.1 7.2 7.3 7.4 Centers for Disease Control and Prevention (22 October 2018). "Lymphatic Filariasis". Parasites. https://www.cdc.gov/parasites/lymphaticfilariasis/.
- ↑ "Lymphatic filariasis". Health Topics A to Z. World Health Organization. http://allcountries.org/health/lymphatic_filariasis.html.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 "Lymphatic filariasis" (in en). https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis.
- ↑ 10.0 10.1 "Working to overcome the global impact of neglected tropical diseases – Summary". Relevé Épidémiologique Hebdomadaire 86 (13): 113–20. March 2011. PMID 21438440. https://www.who.int/wer/2011/wer8613.pdf?ua=1.
- ↑ 11.0 11.1 11.2 "Global programme to eliminate lymphatic filariasis: progress report, 2022" (in en). https://www.who.int/publications-detail-redirect/who-wer9841-489-502.
- ↑ 12.0 12.1 "Filariasis and lymphoedema". Parasite Immunology 31 (11): 664–72. November 2009. doi:10.1111/j.1365-3024.2009.01133.x. PMID 19825106.
- ↑ "Review article: the diagnostic approach and current management of chylous ascites". Alimentary Pharmacology & Therapeutics 46 (9): 816–24. November 2017. doi:10.1111/apt.14284. PMID 28892178.
- ↑ James, William D. et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 978-0-7216-2921-6.
- ↑ Prevention, CDC-Centers for Disease Control and (2020-09-17). "CDC - Lymphatic Filariasis - General Information - Frequently Asked Questions" (in en-us). https://www.cdc.gov/parasites/lymphaticfilariasis/gen_info/faqs.html.
- ↑ Jha, Suman K.; Karna, Bibek; Mahajan, Kunal (2020), "Tropical Pulmonary Eosinophilia", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 32491456, http://www.ncbi.nlm.nih.gov/books/NBK557524/, retrieved 2020-12-01
- ↑ "Pulmonary Eosinophilia". http://emedicine.medscape.com/article/301070-overview. Retrieved 2011-04-16.
- ↑ "ICD-10 Classification". http://apps.who.int/classifications/apps/icd/icd10online/?gj80.htm+j82. Retrieved 2011-04-16.
- ↑ Saladin, Kenneth (2007). Anatomy & Physiology: The Unity of Form and Function. McGraw-Hill. ISBN 978-0-07-287506-5.
- ↑ "A rapid, simple method for isolation of viable microfilariae". Am J Trop Med Hyg 35 (1): 148–51. 1986. doi:10.4269/ajtmh.1986.35.148. PMID 3456213.
- ↑ "A polymerase chain reaction assay for detection of the parasite Wuchereria bancrofti in human blood samples". Am J Trop Med Hyg 54 (4): 357–63. 1996. doi:10.4269/ajtmh.1996.54.357. PMID 8615447.
- ↑ "Live adult worms detected by ultrasonography in human Bancroftian filariasis". Am J Trop Med Hyg 50 (6): 753–7. 1994. doi:10.4269/ajtmh.1994.50.753. PMID 8024070.
- ↑ 23.0 23.1 23.2 "Podoconiosis, a neglected tropical disease". The Netherlands Journal of Medicine 70 (5): 210–14. 2012. PMID 22744921. https://www.njmonline.nl/getpdf.php?id=1182.
- ↑ "Podoconiosis: non-infectious geochemical elephantiasis". Transactions of the Royal Society of Tropical Medicine and Hygiene 101 (12): 1175–80. 2007. doi:10.1016/j.trstmh.2007.08.013. PMID 17976670.
- ↑ Swales, Jay (2006). "Malaria: Fever Wars". CDC. https://www.cdc.gov/malaria/malaria_worldwide/reduction/itn.html.
- ↑ 26.0 26.1 26.2 The Carter Center. "Lymphatic Filariasis Elimination Program". http://www.cartercenter.org/resources/pdfs/factsheets/lymphatic-filariasis-facts.pdf.
- ↑ U.S. Centers for Disease Control and Prevention. "Lymphatic". https://www.cdc.gov/ncidod/dpd/parasites/lymphaticfilariasis/prevention_lymphatic_filar.htm.
- ↑ World Health Organization (31 July 2017). "Congratulations, Tonga! Pacific island state eliminates lymphatic filariasis as a public health problem". https://www.who.int/westernpacific/news/item/31-07-2017-congratulations-tonga-pacific-island-state-eliminates-lymphatic-filariasis-as-a-public-health-problem.
- ↑ World Health Organization (29 October 2020). "Lymphatic filariasis: reporting continued progress towards elimination as a public health problem". News. World Health Organization. https://www.who.int/news/item/29-10-2020-lymphatic-filariasis-reporting-continued-progress-towards-elimination-as-a-public-health-problem.
- ↑ "Multivalent fusion protein vaccine for lymphatic filariasis". Vaccine 31 (12): 1616–22. March 2013. doi:10.1016/j.vaccine.2012.09.055. PMID 23036503.
- ↑ "Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes". PLOS Pathogens 7 (11): e1002351. November 2011. doi:10.1371/journal.ppat.1002351. PMID 22072969.
- ↑ "Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis". Parasitology 141 (1): 119–27. 2014. doi:10.1017/s0031182013001108. PMID 23866958.
- ↑ Prevention, CDC-Centers for Disease Control and (2020-10-26). "CDC - Lymphatic Filariasis - Resources for Health Professionals - Guidance for Evaluation and Treatment" (in en-us). https://www.cdc.gov/parasites/lymphaticfilariasis/health_professionals/dxtx.html.
- ↑ "Lymphatic filariasis" (in en). https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis.
- ↑ Brown, K. R.; Ricci, F. M.; Ottesen, E. A. (2000). "Ivermectin: effectiveness in lymphatic filariasis". Parasitology 121 Suppl: S133–146. doi:10.1017/s0031182000006570. ISSN 0031-1820. PMID 11386685. https://pubmed.ncbi.nlm.nih.gov/11386685/.
- ↑ "The management of lymphatic disorders". Vascular surgery (4th ed.). Philadelphia: WB Saunders. 1995. pp. 1883–1945.
- ↑ Prevention, CDC-Centers for Disease Control and (2021-08-26). "CDC - Lymphatic Filariasis - Epidemiology & Risk Factors" (in en-us). https://www.cdc.gov/parasites/lymphaticfilariasis/epi.html.
- ↑ G.T. Strickland, ed (2000). "Filariasis". Hunter's tropical medicine and emerging infectious diseases (8th ed.). Philadelphia: E.B. Saunders. pp. 740–53. ISBN 978-0-7216-6223-7.
- ↑ 39.0 39.1 Fontes, Gilberto; da Rocha, Eliana Maria Mauricio; Scholte, Ronaldo Guilherme Carvalho; Nicholls, Rubén Santiago (2020-12-22). "Progress towards elimination of lymphatic filariasis in the Americas region". International Health 13 (Suppl 1): S33–S38. doi:10.1093/inthealth/ihaa048. ISSN 1876-3405. PMID 33349876.
- ↑ Fontes, G.; Rocha, E. M.; Brito, A. C.; Fireman, F. A.; Antunes, C. M. (June 2000). "The microfilarial periodicity of Wuchereria bancrofti in north-eastern Brazil". Annals of Tropical Medicine and Parasitology 94 (4): 373–379. doi:10.1080/00034983.2000.11813552. ISSN 0003-4983. PMID 10945047. https://pubmed.ncbi.nlm.nih.gov/10945047/.
- ↑ Fontes, Gilberto; Leite, Anderson Brandão; de Lima, Ana Rachel Vasconcelos; Freitas, Helen; Ehrenberg, John Patrick; da Rocha, Eliana Maria Mauricio (2012-11-26). "Lymphatic filariasis in Brazil: epidemiological situation and outlook for elimination". Parasites & Vectors 5: 272. doi:10.1186/1756-3305-5-272. ISSN 1756-3305. PMID 23181663.
- ↑ McPherson, T (2003-01-24). "Impact on the quality of life of lymphoedema patients following introduction of a hygiene and skin care regimen in a Guyanese community endemic for lymphatic filariasis: A preliminary clinical intervention study". Filaria Journal 2 (1): 1. doi:10.1186/1475-2883-2-1. ISSN 1475-2883. PMID 12605723.
- ↑ "Ten years of podoconiosis research in Ethiopia". PLOS Neglected Tropical Diseases 7 (10): e2301. 2013. doi:10.1371/journal.pntd.0002301. PMID 24130908.
- ↑ The Carter Center. "Lymphatic Filariasis Elimination Program". http://www.cartercenter.org/health/lf/index.html.
- ↑ 45.0 45.1 45.2 "Lymphatic Filariasis Discovery". http://www.stanford.edu/class/humbio103/ParaSites2006/Lymphatic_filariasis/Discovery.htm.
- ↑ "HYMN L. Various Deities". https://www.sacred-texts.com/hin/rigveda/rv07050.htm.
- ↑ Otsuji, Y. (2011). "History, Epidemiology and Control of Filariasis". Tropical Medicine and Health 39 (1 Suppl 2): 3–13. doi:10.2149/tmh.39-1-suppl_2-3. PMID 22028595.
- ↑ Grove, David I (1990). A history of human helminthology. Wallingford: CAB International. pp. 1–848. ISBN 0-85198-689-7.
- ↑ Grove, David I (2014). Tapeworms, lice and prions: a compendium of unpleasant infections. Oxford: Oxford University Press. pp. 1–602. ISBN 978-0-19-964102-4.
- ↑ Burma D.P. (2010). Project Of History Of Science, Philosophy And Culture In Indian Civilization, Volume Xiii Part 2: From Physiology And Chemistry To Biochemistry. Pearson Education India. p. 49. ISBN 978-81-317-3220-5. https://books.google.com/books?id=4CaQ3-x3LXMC.
- ↑ "Multivalent fusion protein vaccine for lymphatic filariasis". Vaccine 31 (12): 1616–22. March 2013. doi:10.1016/j.vaccine.2012.09.055. PMID 23036503. (primary source)
- ↑ "Draft genome of the filarial nematode parasite Brugia malayi". Science 317 (5845): 1756–60. September 2007. doi:10.1126/science.1145406. PMID 17885136. Bibcode: 2007Sci...317.1756G.
- ↑ "The filarial genome project: analysis of the nuclear, mitochondrial and endosymbiont genomes of Brugia malayi". International Journal for Parasitology 30 (4): 411–19. April 2000. doi:10.1016/s0020-7519(00)00014-x. PMID 10731564.
- ↑ Bain, Odile (2002). "Evolutionary Relationships Among Filarial Nematodes". World Class Parasites. 5. Boston: Kluwer. pp. 21–9. doi:10.1007/0-306-47661-4_3. ISBN 1-4020-7038-1. ISBN 978-1-4020-7038-9. ISBN 978-1-4757-7600-3. ISBN 978-0-306-47661-7.
- ↑ Taylor, Mark J.; Bandi, Claudio; Hoerauf, Achim (2005). "Wolbachia Bacterial Endosymbionts of Filarial Nematodes". Advances in Parasitology. 60. Elsevier. pp. 245–84. doi:10.1016/s0065-308x(05)60004-8. ISBN 9780120317608.
External links
| Classification | |
|---|---|
| External resources |
 KSF
KSF