Plasminogen activator
Topic: Medicine
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
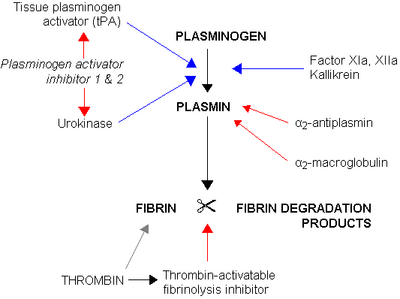
Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.[1]
Plasminogen activators are inhibited by plasminogen activator inhibitor-1, plasminogen activator inhibitor-2, and protein C inhibitor.
Function
Produced mainly in the liver, plasminogen is the inactive zymogen form of plasmin, and circulates in plasma in a closed conformation that cannot be activated. Binding clots or cell surface causes its conformation to change, allowing it to be activated by plasminogen activators. Plasminogen activators do so by cleaving the R561/V562 peptide bond, producing the active protein plasmin, which catalyzes the degradation of fibrin polymers that make up the structure of blood clots.[2]
Inhibition
The main inhibitor of tissue plasminogen activator and urokinase is plasminogen activator inhibitor-1 (PAI-1).[3] Plasminogen activator inhibitor-1 is a serine protease, synthesized by endothelial cells, that specifically inhibits tissue plasminogen activator (tPA) and urokinase (uPA). Tissue plasminogen activator and urokinase are the activators of plasminogen and results in the breakdown of blood clots (fibrinolysis).[4]
PAI-1 levels has also been studied in patients and how they influence certain diseases. Elevated serum levels of PAI-1 have been found in obese individuals.[5] Elevated levels of PAI-1 also seem to increase the risk of atherothrombotic events and may also promote vascular disease.[6]
Plasminogen activator inhibitor-2 (PAI-2) is also a serine protease that inactivates tPA and uPA. PAI-2 is produced by the placenta and only found in high quantities in the blood during pregnancy.[7]
Factors
Factor XIa and XIIa are two main factors involved in the plasminogen activator. Factor XI (FXI) is a serine protase produced by the liver and circulates in its inactive form.[8] Deficiency in factor XI is known to cause hemophilia C.[9] Factor XIIa is another plasma protein that is involved in the activation of zymogen factor is activated into factor XIa.[10] This activation is important to the coagulation cascade.
Applications
Due to its contribution to fibrinolysis, tissue plasminogen activator is used medically to treat blood clot-related disorders including thrombotic or embolic stroke, myocardial infarction, and pulmonary embolism. It is manufactured using recombinant techniques and is sold as alteplase, reteplase, and tenecteplase. Alteplase was the first of these versions to go on the market, and has the same exact structure as tPA. Reteplase and tenecteplase both received FDA approval after alteplase, and have nonidentical structures to tPA.[1] These recombinant forms of tPA have been shown to have a longer half-life in the blood and a greater resistance to inhibition, resulting in an increased capacity to treat thrombolytic diseases.[11]
Urokinase is similarly used in the medical field, specifically for the treatment of pulmonary embolism.[12]
Plasminogen Activator Role in Breast Cancer
An Error has occurred retrieving Wikidata item for infoboxPlasminogen activator inhibitor-1 not only functions as an inhibitor, but other roles of PAI-1 could suggest it could contribute to cancer. The other roles of PAI-1 include, cell de-adhesion, cell proliferation, apoptosis, and cell signaling. These roles could suggest that PAI-1 expression in the tumor microenvironment enhances tumor cell progression. Urokinase cleaves the zymogen plasminogen into serine protease plasmin. The elevated levels of uPA is an indicator of cancer which could be found in the carcinoma of the breast. Plasmin can activate matrix metalloproteases (MMP's) in the extracellular matrix (ECM). MMP activation contributes to tumor cell invasion and metastasis by degradation of ECM components.[5]
References
- ↑ 1.0 1.1 Rivera-Bou, Wanda L (15 December 2016). "Thrombolytic Therapy". http://emedicine.medscape.com/article/811234-overview#a2.
- ↑ "The X-ray crystal structure of full-length human plasminogen". Cell Reports 1 (3): 185–90. March 2012. doi:10.1016/j.celrep.2012.02.012. PMID 22832192.
- ↑ "Three decades of research on plasminogen activator inhibitor-1: a multifaceted serpin". Seminars in Thrombosis and Hemostasis 39 (4): 356–64. June 2013. doi:10.1055/s-0033-1334487. PMID 23504606.
- ↑ "[Type 1 plasminogen activator inhibitor: its role in biological reactions]". [Rinsho Ketsueki] the Japanese Journal of Clinical Hematology 32 (5): 487–9. May 1991. PMID 1870265.
- ↑ 5.0 5.1 "Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-γ and plasminogen activator inhibitor-1". PPAR Research 2009: 345320. 2009-01-01. doi:10.1155/2009/345320. PMID 19672469.
- ↑ "PAI-1 and atherothrombosis". Journal of Thrombosis and Haemostasis 3 (8): 1879–83. August 2005. doi:10.1111/j.1538-7836.2005.01420.x. PMID 16102055.
- ↑ "Plasminogen-activator inhibitor type 2 (PAI-2) is a spontaneously polymerising SERPIN. Biochemical characterisation of the recombinant intracellular and extracellular forms". European Journal of Biochemistry 218 (3): 1071–82. December 1993. doi:10.1111/j.1432-1033.1993.tb18467.x. PMID 7506655.
- ↑ "Factor XI homodimer structure is essential for normal proteolytic activation by factor XIIa, thrombin, and factor XIa". The Journal of Biological Chemistry 283 (27): 18655–64. July 2008. doi:10.1074/jbc.M802275200. PMID 18441012.
- ↑ "Factor XI deficiency". Baillière's Clinical Haematology 9 (2): 355–68. June 1996. doi:10.1016/s0950-3536(96)80068-0. PMID 8800510.
- ↑ "Roles of platelets and factor XI in the initiation of blood coagulation by thrombin". Thrombosis and Haemostasis 86 (1): 75–82. July 2001. doi:10.1055/s-0037-1616203. PMID 11487044.
- ↑ "Recombinant tissue plasminogen activators (rtPA): a review". Clinical Pharmacology and Therapeutics 97 (3): 274–85. March 2015. doi:10.1002/cpt.33. PMID 25670034.
- ↑ "LABEL: KINLYTIC- urokinase injection, powder, lyophilized, for solution". 8 June 2007. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7966.
External links
- Overview of all the structural information available in the PDB for UniProt: P00747 (Plasminogen) at the PDBe-KB.
 |
 KSF
KSF