Stomach cancer
Topic: Medicine
 From HandWiki - Reading time: 23 min
From HandWiki - Reading time: 23 min
| Stomach cancer | |
|---|---|
| Other names | Gastric cancer |
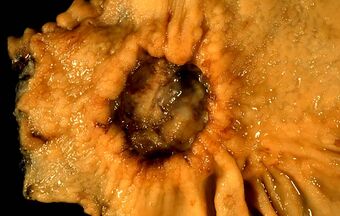 | |
| A stomach ulcer that was diagnosed as cancer on biopsy and surgically removed | |
| Specialty | Gastroenterology Oncology |
| Symptoms | Early:
|
| Usual onset | Over years[2] |
| Types | Gastric carcinomas, lymphoma, mesenchymal tumor[2] |
| Causes | Helicobacter pylori, genetics[2][3] |
| Risk factors | Smoking, dietary factors such as pickled vegetables, obesity[2][4] |
| Diagnostic method | Biopsy done during endoscopy[1] |
| Prevention | Mediterranean diet, not smoking[2][5] |
| Treatment | Surgery, chemotherapy, radiation therapy, targeted therapy[1] |
| Prognosis | Five-year survival rate: < 10% (advanced cases),[6] 32% (US),[7] 71% (Japan)[8] |
| Frequency | 3.5 million (2015)[9] |
| Deaths | 783,000 (2018)[10] |
Stomach cancer, also known as gastric cancer, is a cancer that develops from the lining of the stomach.[11] Most cases of stomach cancers are gastric carcinomas, which can be divided into a number of subtypes, including gastric adenocarcinomas.[2] Lymphomas and mesenchymal tumors may also develop in the stomach.[2] Early symptoms may include heartburn, upper abdominal pain, nausea, and loss of appetite.[1] Later signs and symptoms may include weight loss, yellowing of the skin and whites of the eyes, vomiting, difficulty swallowing, and blood in the stool, among others.[1] The cancer may spread from the stomach to other parts of the body, particularly the liver, lungs, bones, lining of the abdomen, and lymph nodes.[12]
The most common cause is infection by the bacterium Helicobacter pylori, which accounts for more than 60% of cases.[2][3][13] Certain strains of H. pylori have greater risks than others.[2] Smoking, dietary factors such as pickled vegetables and obesity are other risk factors.[2][4] About 10% of cases run in families, and between 1% and 3% of cases are due to genetic syndromes inherited such as hereditary diffuse gastric cancer.[2] Most of the time, stomach cancer develops in stages over years.[2] Diagnosis is usually by biopsy done during endoscopy.[1] This is followed by medical imaging to determine if the disease has spread to other parts of the body.[1] Japan and South Korea , two countries that have high rates of the disease, screen for stomach cancer.[2]
A Mediterranean diet lowers the risk of stomach cancer, as does not smoking.[2][5] Tentative evidence indicates that treating H. pylori decreases the future risk.[2][5] If stomach cancer is treated early, it can be cured.[2] Treatments may include some combination of surgery, chemotherapy, radiation therapy, and targeted therapy.[1][14] For certain subtypes of gastric cancer, cancer immunotherapy is an option as well.[15] If treated late, palliative care may be advised.[2] Some types of lymphoma can be cured by eliminating H. pylori.[16] Outcomes are often poor, with a less than 10% five-year survival rate in the Western world for advanced cases.[6] This is largely because most people with the condition present with advanced disease.[6] In the United States, five-year survival is 31.5%,[7] while in South Korea it is over 65% and Japan over 70%, partly due to screening efforts.[2][8]
Globally, stomach cancer is the fifth-leading type of cancer and the third-leading cause of death from cancer, making up 7% of cases and 9% of deaths.[17] In 2018, it newly occurred in 1.03 million people and caused 783,000 deaths.[10] Before the 1930s, it was a leading cause of cancer deaths in the Western world, however rates have sharply declined among younger generations in the West, while they remain high for people living in East Asia.[18][19][20] The decline in the West is believed to be due to the decline of salted and pickled food consumption, as a result of the development of refrigeration as a method of preserving food.[21] Stomach cancer occurs most commonly in East Asia, followed by Eastern Europe.[2] It occurs twice as often in males as in females.[2]
Signs and symptoms
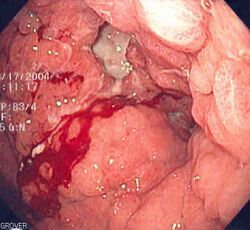
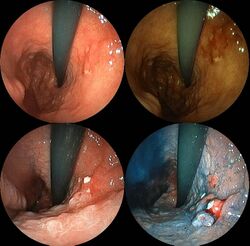
Stomach cancer is often either asymptomatic (producing no noticeable symptoms) or it may cause only nonspecific symptoms (which may also be present in other related or unrelated disorders) in its early stages. By the time symptoms are recognized, the cancer has often reached an advanced stage (see below) and may have metastasized (spread to other, perhaps distant, parts of the body), which is one of the main reasons for its relatively poor prognosis.[22] Stomach cancer can cause the following signs and symptoms: Unexplained nausea, vomiting, diarrhoea and constipation. Patients also can experience unexplained weight loss.[23]
Early cancers may be associated with indigestion or a burning sensation (heartburn). However, fewer than one in every 50 people referred for endoscopy due to indigestion has cancer.[24] Abdominal discomfort and loss of appetite, especially for meat, can occur.[citation needed]
Gastric cancers that have enlarged and invaded normal tissue can cause weakness, fatigue, bloating of the stomach after meals, abdominal pain in the upper abdomen, nausea and occasional vomiting. Further enlargement may cause weight loss or bleeding with vomiting blood or having blood in the stool, the latter apparent as black discolouration (melena) and sometimes leading to anemia. Dysphagia suggests a tumour in the cardia or extension of the gastric tumour into the esophagus.[citation needed]
These can be symptoms of other problems such as a stomach virus, gastric ulcer, or tropical sprue.[citation needed]
Risk factors
Gastric cancer can occur as a result of many factors.[25] It occurs twice as commonly in males as females. Estrogen may protect women against the development of this form of cancer.[26][27]
Infections
Helicobacter pylori infection is an essential risk factor in 65–80% of gastric cancers, but only 2% of people with H. pylori infections develop stomach cancer.[4][28] The mechanism by which H. pylori induces stomach cancer potentially involves chronic inflammation, the action of H. pylori virulence factors such as CagA,[29] or an interaction between H. pylori infection and germline pathogenic variants in homologous-recombination genes.[30] It was estimated that Epstein–Barr virus is responsible for 84,000 cases per year.[31] AIDS is also associated with elevated risk.[4]
Smoking
Smoking increases the risk of developing gastric cancer significantly, from 40% increased risk for current smokers to 82% increase for heavy smokers. Gastric cancers due to smoking mostly occur in the upper part of the stomach near the esophagus.[32][33][34]
Alcohol
Some studies show increased risk with alcohol consumption as well.[4][35]
Diet

Dietary factors are not proven causes, and the association between stomach cancer and various foods and beverages is weak.[37] Some foods including fried foods,[38] smoked foods, salt and salt-rich foods, meat,[39] processed meat,[39] red meat,[39] pickled vegetables, and brackens[40] are associated with a higher risk of stomach cancer.[4][41]
Fresh fruit and vegetable intake,[42] citrus fruit intake,[42] and antioxidant intake are associated with a lower risk of stomach cancer.[4][32] A Mediterranean diet is associated with lower rates of stomach cancer,[43] as is regular aspirin use.[4]
Obesity is a physical risk factor that has been found to increase the risk of gastric adenocarcinoma by contributing to the development of gastroesophageal reflux disease (GERD).[44] The exact mechanism by which obesity causes GERD is not completely known. Studies hypothesize that increased dietary fat leading to increased pressure on the stomach and the lower esophageal sphincter, due to excess adipose tissue, could play a role, yet no statistically significant data have been collected.[45] However, the risk of gastric cardia adenocarcinoma, with GERD present, has been found to increase more than 2 times for an obese person.[44] There is a correlation between iodine deficiency and gastric cancer.[46][47][48]
Genetics
About 10% of cases run in families, and between 1 and 3% of cases are due to genetic syndromes inherited such as hereditary diffuse gastric cancer.[2]
A genetic risk factor for gastric cancer is a genetic defect of the CDH1 gene known as hereditary diffuse gastric cancer (HDGC). The CDH1 gene, which codes for E-cadherin, lies on the 16th chromosome.[49] When the gene experiences a particular mutation, gastric cancer develops through a mechanism that is not fully understood.[49][50] This mutation is considered autosomal dominant, meaning that half of a carrier's children will likely experience the same mutation.[49] Diagnosis of hereditary diffuse gastric cancer usually takes place when at least two cases involving a family member, such as a parent or grandparent, are diagnosed, with at least one diagnosed before the age of 50.[49] The diagnosis can also be made if at least three cases occur in the family, in which case age is not considered.[49]
The International Cancer Genome Consortium is leading efforts to identify genomic changes involved in stomach cancer.[51][52] A very small percentage of diffuse-type gastric cancers (see Histopathology below) arise from an inherited abnormal CDH1 gene. Genetic testing and treatment options are available for families at risk.[53]
Other
Other risk factors include diabetes,[54] pernicious anemia,[35] chronic atrophic gastritis,[55] Menetrier's disease (hyperplastic, hypersecretory gastropathy),[56] and intestinal metaplasia.[57]
In addition, Foxp3 polymorphism (rs3761548) might contribute to gastric cancer development through influencing Treg cell activity.[58]
In a human retrospective study, biliary reflux was found to be a likely risk factor for gastric cancer and precancerous lesions.[59]
Diagnosis
To find the cause of symptoms, the doctor asks about the patient's medical history, does a physical examination, and may order laboratory studies.[60] The patient may also have one or all of these exams:
- Gastroscopic exam is the diagnostic method of choice. This involves insertion of a fibre optic camera into the stomach to visualise it.[35]
- Upper GI series (may be called barium roentgenogram)
- Computed tomography or CT scanning of the abdomen may reveal gastric cancer. It is more useful to determine invasion into adjacent tissues or the presence of spread to local lymph nodes. Wall thickening of more than 1 cm that is focal, eccentric, and enhancing favours malignancy.[61]
In 2013, Chinese and Israeli scientists reported a successful pilot study of a breathalyzer-style breath test intended to diagnose stomach cancer by analyzing exhaled chemicals without the need for an intrusive endoscopy.[62][63] A larger-scale clinical trial of this technology was completed in 2014.[64][65]
Abnormal tissue seen in a gastroscope examination is biopsied by the surgeon or gastroenterologist. This tissue is then sent to a pathologist for histological examination under a microscope to check for the presence of cancerous cells. A biopsy, with subsequent histological analysis, is the only sure way to confirm the presence of cancer cells.[35]
Various gastroscopic modalities have been developed to increase yield of detected mucosa with a dye that accentuates the cell structure and can identify areas of dysplasia. Endocytoscopy involves ultra-high magnification to visualise cellular structure to better determine areas of dysplasia. Other gastroscopic modalities such as optical coherence tomography are being tested investigationally for similar applications.[66]
A number of cutaneous conditions are associated with gastric cancer. A condition of darkened hyperplasia of the skin, frequently of the axilla and groin, known as acanthosis nigricans, is associated with intra-abdominal cancers such as gastric cancer. Other cutaneous manifestations of gastric cancer include "tripe palms" (a similar darkening hyperplasia of the skin of the palms) and the Leser-Trelat sign, which is the rapid development of skin lesions known as seborrheic keratoses.[67]
Various blood tests may be done, including a complete blood count to check for anaemia, and a fecal occult blood test to check for blood in the stool.[68]
Histopathology
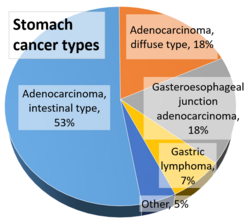
- Gastric adenocarcinoma is a malignant epithelial tumour, originating from glandular epithelium of the gastric mucosa. Stomach cancers are about 90% adenocarcinomas.[70] Histologically, there are two major types of gastric adenocarcinoma (Lauren classification): intestinal type or diffuse type. Adenocarcinomas tend to aggressively invade the gastric wall, infiltrating the muscularis mucosae, the submucosa and then the muscularis propria. Intestinal type adenocarcinoma tumour cells describe irregular tubular structures, harbouring pluristratification, multiple lumens, reduced stroma ("back to back" aspect). Often, it associates intestinal metaplasia in neighbouring mucosa. Depending on glandular architecture, cellular pleomorphism and mucosecretion, adenocarcinoma may present 3 degrees of differentiation: well, moderate and poorly differentiated. Diffuse type adenocarcinoma (mucinous, colloid, linitis plastica or leather-bottle stomach) tumour cells are discohesive and secrete mucus, which is delivered in the interstitium, producing large pools of mucus/colloid (optically "empty" spaces). It is poorly differentiated. In signet-ring cell carcinomas, the mucus remains inside the tumour cell and pushes the nucleus to the periphery, giving rise to signet-ring cells.[citation needed]
- Around 5% of gastric cancers are lymphomas.[71] These may include extranodal marginal zone B-cell lymphomas (MALT type)[72] and to a lesser extent diffuse large B-cell lymphomas.[73] MALT type make up about half of stomach lymphomas.[16]
- Carcinoid and stromal tumors may occur.[citation needed]
-
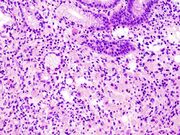
Poor to moderately differentiated adenocarcinoma of the stomach. H&E stain.
-
Gastric signet ring cell carcinoma. H&E stain.
-
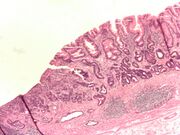
Adenocarcinoma of the stomach and intestinal metaplasia. H&E stain.
Staging

If cancer cells are found in the tissue sample, the next step is to stage, or find out the extent of the disease. Various tests determine whether the cancer has spread, and if so, what parts of the body are affected. Because stomach cancer can spread to the liver, pancreas, and other organs near the stomach, as well as to the lungs, the doctor may order a CT scan, a PET scan,[74] an endoscopic ultrasound exam, or other tests to check these areas. Blood tests for tumor markers, such as carcinoembryonic antigen and carbohydrate antigen may be ordered, as their levels correlate to extent of metastasis, especially to the liver, and the cure rate.[citation needed]
Staging may not be complete until after surgery. The surgeon removes nearby lymph nodes and possibly samples of tissue from other areas in the abdomen for examination by a pathologist.[citation needed]
The clinical stages of stomach cancer are:[75][76]
- Stage 0 – Limited to the inner lining of the stomach, it is treatable by endoscopic mucosal resection when found very early (in routine screenings), or otherwise by gastrectomy and lymphadenectomy without need for chemotherapy or radiation.
- Stage I – Penetration to the second or third layers of the stomach (stage 1A) or to the second layer and nearby lymph nodes (stage 1B): Stage 1A is treated by surgery, including removal of the omentum. Stage 1B may be treated with chemotherapy (5-fluorouracil) and radiation therapy.
- Stage II – Penetration to the second layer and more distant lymph nodes, or the third layer and only nearby lymph nodes, or all four layers but not the lymph nodes, it is treated as for stage I, sometimes with additional neoadjuvant chemotherapy.
- Stage III – Penetration to the third layer and more distant lymph nodes, or penetration to the fourth layer and either nearby tissues or nearby or more distant lymph nodes, it is treated as for stage II; a cure is still possible in some cases.
- Stage IV – Cancer has spread to nearby tissues and more distant lymph nodes, or has metastasized to other organs. A cure is very rarely possible at this stage. Some other techniques to prolong life or improve symptoms are used, including laser treatment, surgery, and/or stents to keep the digestive tract open, and chemotherapy by drugs such as 5-fluorouracil, cisplatin, epirubicin, etoposide, docetaxel, oxaliplatin, capecitabine, or irinotecan.[14]
The TNM staging system is also used.[77]
In a study of open-access endoscopy in Scotland, patients were diagnosed 7% in stage I, 17% in stage II, and 28% in stage III.[78] A Minnesota population was diagnosed 10% in stage I, 13% in stage II, and 18% in stage III.[79] However, in a high-risk population in the Valdivia Province of southern Chile , only 5% of patients were diagnosed in the first two stages and 10% in stage III.[80]
Prevention
Getting rid of H. pylori in those who are infected decreases the risk of stomach cancer.[81] A 2014 meta-analysis of observational studies found that a diet high in fruits, mushrooms, garlic, soybeans, and green onions was associated with a lower risk of stomach cancer in the Korean population.[82] Low doses of vitamins, especially from a healthy diet, decrease the risk of stomach cancer.[83] A previous review of antioxidant supplementation did not find supporting evidence and possibly worse outcomes.[84][85] Modern technology is used to promote early diagnosis, e.g. based on serum markers.[86]
Management
Cancer of the stomach is difficult to cure unless it is found at an early stage (before it has begun to spread). Unfortunately, because early stomach cancer causes few symptoms, the disease is usually advanced when the diagnosis is made.[87]
Treatment for stomach cancer may include surgery,[88] chemotherapy,[14] or radiation therapy.[89] New treatment approaches such as immunotherapy or gene therapy and improved ways of using current methods are being studied in clinical trials.[90]
Surgery
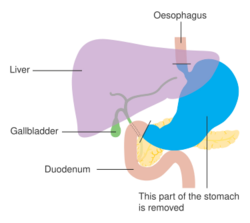
Surgery remains the only curative therapy for stomach cancer.[6] Of the different surgical techniques, endoscopic mucosal resection (EMR) is a treatment for early gastric cancer (tumor only involves the mucosa) that was pioneered in Japan and is available in the United States at some centers.[6] In EMR, the tumor, together with the inner lining of stomach (mucosa), is removed from the wall of the stomach using an electrical wire loop through the endoscope. The advantage is that it is a much smaller operation than removing the stomach.[6] Endoscopic submucosal dissection is a similar technique pioneered in Japan, used to resect a large area of mucosa in one piece.[6] If the pathologic examination of the resected specimen shows incomplete resection or deep invasion by tumor, the patient would need a formal stomach resection.[6] A 2016 Cochrane review found low-quality evidence of no difference in short-term mortality between laparoscopic and open gastrectomy (removal of stomach), and that benefits or harms of laparoscopic gastrectomy cannot be ruled out.[91] Post-operatively, up to 70% of people undergoing total gastrectomy develop complications such as dumping syndrome and reflux esophagitis.[92] Construction of a "pouch", which serves as a "stomach substitute", reduced the incidence of dumping syndrome and reflux esophagitis by 73% and 63% respectively, and led to improvements in quality-of-life, nutritional outcomes, and body mass index.[92]
Those with metastatic disease at the time of presentation may receive palliative surgery, and while it remains controversial, due to the possibility of complications from the surgery itself and because it may delay chemotherapy, the data so far are mostly positive, with improved survival rates being seen in those treated with this approach.[6][93]
Chemotherapy
The use of chemotherapy to treat stomach cancer has no firmly established standard of care.[14] Unfortunately, stomach cancer has not been particularly sensitive to these drugs, and chemotherapy, if used, has usually served to palliatively reduce the size of the tumor, relieve symptoms of the disease, and increase survival time.[14] Some drugs used in stomach cancer treatment have included: fluorouracil or its analog capecitabine, BCNU (carmustine), methyl-CCNU (semustine) and doxorubicin (Adriamycin), as well as mitomycin C, and more recently cisplatin and taxotere, often using drugs in various combinations.[14] The relative benefits of these different drugs, alone and in combination, are unclear.[14][94] Clinical researchers are exploring the benefits of giving chemotherapy before surgery to shrink the tumor, or as adjuvant therapy after surgery to destroy remaining cancer cells.[6]
Targeted therapy
Recently[when?], treatment with human epidermal growth factor receptor 2 (HER2) inhibitor, trastuzumab, has been demonstrated to increase overall survival in inoperable locally advanced or metastatic gastric carcinoma over-expressing the HER2/neu gene.[6] In particular, HER2 is overexpressed in 13–22% of patients with gastric cancer.[90][95] Of note, HER2 overexpression in gastric neoplasia is heterogeneous and comprises a minority of tumor cells (less than 10% of gastric cancers overexpress HER2 in more than 5% of tumor cells). Hence, this heterogeneous expression should be taken into account for HER2 testing, particularly in small samples such as biopsies, requiring the evaluation of more than one bioptic sample.[95]
Radiation
Radiation therapy (also called radiotherapy) may be used to treat stomach cancer, often as an adjuvant to chemotherapy and/or surgery.[6]
Lymphoma
MALT lymphomas are often completely resolved after the underlying H. pylori infection is treated.[16] This results in remission in about 80% of cases.[16]
Prognosis
The prognosis of stomach cancer is generally poor, because the tumor has often metastasized by the time of discovery, and most people with the condition are elderly (median age is between 70 and 75 years) at presentation.[96] The average life expectancy after being diagnosed is around 24 months, and the five-year survival rate for stomach cancer is less than 10%.[6]
Almost 300 genes are related to outcomes in stomach cancer, with both unfavorable genes where high expression is related to poor survival and favorable genes where high expression is associated with longer survival times.[97][98] Examples of poor prognosis genes include ITGAV, DUSP1 and P2RX7.[99]
Epidemiology
In 2018, stomach cancer was the fifth most frequently diagnosed cancer worldwide, representing 5.7% of all cancer cases, and the third leading cause of death from cancers, being responsible for 8.2% of all cancer deaths.[100] Among men, 683 754 cases were diagnosed, accounting for 7.2% of all cancer cases, and among women, stomach cancer was diagnosed in 349 947 cases, accounting for 4.1% of all cancer cases.[100]
In 2012, stomach cancer was the fifth most-common cancer with 952,000 cases diagnosed.[17] It is more common both in men and in developing countries.[101][102] In 2012, it represented 8.5% of cancer cases in men, making it the fourth most-common cancer in men.[103] Also in 2012, the number of deaths was 700,000, having decreased slightly from 774,000 in 1990, making it the third-leading cause of cancer-related death (after lung cancer and liver cancer).[104][105]
Less than 5% of stomach cancers occur in people under 40 years of age, with 81.1% of that 5% in the age-group of 30 to 39 and 18.9% in the age-group of 20 to 29.[106]
In 2014, stomach cancer resulted in 0.61% of deaths (13,303 cases) in the United States.[107] In China, stomach cancer accounted for 3.56% of all deaths (324,439 cases).[108][unreliable source?] The highest rate of stomach cancer was in Mongolia, at 28 cases per 100,000 people.[109][unreliable source?]
In the United Kingdom, stomach cancer is the 15th most-common cancer (around 7,100 people were diagnosed with stomach cancer in 2011), and it is the 10th most-common cause of cancer-related deaths (around 4,800 people died in 2012).[110]
Incidence and mortality rates of gastric cancer vary greatly in Africa. The GLOBOCAN system is currently the most widely used method to compare these rates between countries, but African incidence and mortality rates are seen to differ among countries, possibly due to the lack of universal access to a registry system for all countries.[111] Variation as drastic as estimated rates from 0.3/100000 in Botswana to 20.3/100000 in Mali have been observed.[111] In Uganda, the incidence of gastric cancer has increased from the 1960s measurement of 0.8/100000 to 5.6/100000.[111] Gastric cancer, though present, is relatively low when compared to countries with high incidence like Japan and China. One suspected cause of the variation within Africa and between other countries is due to different strains of the H. pylori bacteria. The trend commonly seen is that H. pylori infection increases the risk for gastric cancer, but this is not the case in Africa, giving this phenomenon the name the "African enigma".[112] Although this bacterial species is found in Africa, evidence has supported that different strains with mutations in the bacterial genotype may contribute to the difference in cancer development between African countries and others outside the continent.[112] Increasing access to health care and treatment measures have been commonly associated with the rising incidence, though, particularly in Uganda.[111]
Other animals
The stomach is a muscular organ of the gastrointestinal tract that holds food and begins the digestive process by secreting gastric juice. The most common cancers of the stomach are adenocarcinomas, but other histological types have been reported. Signs vary, but may include vomiting (especially if blood is present), weight loss, anemia, and lack of appetite. Bowel movements may be dark and tarry in nature. To determine whether cancer is present in the stomach, special X-rays and/or abdominal ultrasounds may be performed. Gastroscopy, a test using an endoscope to examine the stomach, is a useful diagnostic tool that can also take samples of the suspected mass for histopathological analysis to confirm or rule out cancer. The most definitive method of cancer diagnosis is through open surgical biopsy.[113] Most stomach tumors are malignant with evidence of spread to lymph nodes or liver, making treatment difficult. Except for lymphoma, surgery is the most frequent treatment option for stomach cancers but it is associated with significant risks.[citation needed]
A carcinogenic interaction was demonstrated between bile acids and Helicobacter pylori in a mouse model of gastric cancer.[114][115]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Gastric Cancer Treatment (PDQ®)". 2014-04-17. http://www.cancer.gov/cancertopics/pdq/treatment/gastric/Patient/page1/AllPages.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 "5.4 Stomach Cancer". World Cancer Report 2014. 2014.
- ↑ 3.0 3.1 "Role of bacteria in oncogenesis". Clinical Microbiology Reviews 23 (4): 837–857. October 2010. doi:10.1128/CMR.00012-10. PMID 20930075.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 "Gastric cancer: epidemiologic aspects". Helicobacter 18 (Suppl 1): 34–38. September 2013. doi:10.1111/hel.12082. PMID 24011243.
- ↑ 5.0 5.1 5.2 "Stomach (Gastric) Cancer Prevention (PDQ®)". 2014-02-27. http://www.cancer.gov/cancertopics/pdq/prevention/gastric/HealthProfessional.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 "Treatment of gastric cancer". World Journal of Gastroenterology 20 (7): 1635–1649. February 2014. doi:10.3748/wjg.v20.i7.1635. PMID 24587643.
- ↑ 7.0 7.1 "Cancer of the Stomach - Cancer Stat Facts" (in en). https://seer.cancer.gov/statfacts/html/stomach.html.
- ↑ 8.0 8.1 "がん診療連携拠点病院等院内がん登録生存率集計:[国立がん研究センター がん登録・統計"]. https://ganjoho.jp/reg_stat/statistics/brochure/hosp_c_reg_surv.html.
- ↑ Vos, Theo et al. (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMID 27733282.
- ↑ 10.0 10.1 "Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries". CA: A Cancer Journal for Clinicians 68 (6): 394–424. November 2018. doi:10.3322/caac.21492. PMID 30207593.
- ↑ "Stomach (Gastric) Cancer". January 1980. http://www.cancer.gov/cancertopics/types/stomach.
- ↑ Cancer biology (4th ed.). Oxford University Press. 2007. p. 223. ISBN 9780195175431. https://books.google.com/books?id=PymZ1ORk0TcC&pg=PA223.
- ↑ Issues in public health (2nd ed.). Open University Press. 2011. p. 74. ISBN 9780335244225. https://books.google.com/books?id=Jn8xC6_UW8YC&pg=PA74.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 "Chemotherapy for advanced gastric cancer". The Cochrane Database of Systematic Reviews 2017 (8): CD004064. August 2017. doi:10.1002/14651858.cd004064.pub4. PMID 28850174.
- ↑ "Targeting the Myeloid-Derived Suppressor Cell Compartment for Inducing Responsiveness to Immune Checkpoint Blockade Is Best Limited to Specific Subtypes of Gastric Cancers". Gastroenterology 161 (2): 727. August 2021. doi:10.1053/j.gastro.2021.03.047. PMID 33798523.
- ↑ 16.0 16.1 16.2 16.3 "Treatment of gastric marginal zone lymphoma of MALT type". Expert Opinion on Pharmacotherapy 11 (13): 2141–2152. September 2010. doi:10.1517/14656566.2010.497141. PMID 20586708.
- ↑ 17.0 17.1 "1.1 The global and regional burden of cancer". World Cancer Report 2014. World Health Organization. 2014. ISBN 978-9283204299.
- ↑ Cancer and its management (6th ed.). Wiley-Blackwell. 2010. p. 259. ISBN 9781444306378. https://books.google.com/books?id=9sW0eCoZFt4C&pg=PA259.
- ↑ Handbook of cancer chemotherapy (8th ed.). Wolter Kluwer. 2011. p. 127. ISBN 9781608317820. https://books.google.com/books?id=6Nz_87OLrtcC&pg=PA127.
- ↑ Human Longevity: The Major Determining Factors. Author House. 2010. p. 339. ISBN 9781452067223. https://books.google.com/books?id=HBY_GxOY_6oC&pg=PA339.[self-published source?]
- ↑ Cancer, culture, and communication. Kluwer Academic. 2004. p. 139. ISBN 9780306478857. https://archive.org/details/springer_10.1007-b105731.
- ↑ "Statistics and outlook for stomach cancer". Cancer Research UK. http://www.cancerresearchuk.org/cancer-help/type/stomach-cancer/treatment/statistics-and-outlook-for-stomach-cancer.
- ↑ Al-Azri, Mohammed; Al-Kindi, Jamila; Al-Harthi, Thuraiya; Al-Dahri, Manal; Panchatcharam, Sathiya Murthi; Al-Maniri, Abdullah (June 2019). "Awareness of Stomach and Colorectal Cancer Risk Factors, Symptoms and Time Taken to Seek Medical Help Among Public Attending Primary Care Setting in Muscat Governorate, Oman" (in en). Journal of Cancer Education 34 (3): 423–434. doi:10.1007/s13187-017-1266-8. ISSN 0885-8195. PMID 28782080. http://link.springer.com/10.1007/s13187-017-1266-8.
- ↑ "Guidance on Commissioning Cancer Services Improving Outcomes in Upper Gastro-intestinal Cancers". NHS. Jan 2001. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4080278.pdf.
- ↑ "Environmental and lifestyle risk factors of gastric cancer". Archives of Iranian Medicine 16 (6): 358–365. June 2013. PMID 23725070. http://www.ams.ac.ir/AIM/NEWPUB/13/16/6/0010.pdf. Retrieved 2019-05-03.
- ↑ "Oestrogen and the enigmatic male predominance of gastric cancer". European Journal of Cancer 44 (16): 2397–2403. November 2008. doi:10.1016/j.ejca.2008.07.031. PMID 18755583.
- ↑ "The direct effect of estrogen on cell viability and apoptosis in human gastric cancer cells". Molecular and Cellular Biochemistry 395 (1–2): 99–107. October 2014. doi:10.1007/s11010-014-2115-2. PMID 24934239.
- ↑ "Proceedings of the fourth Global Vaccine Research Forum". Initiative for Vaccine Research team of the Department of Immunization, Vaccines and Biologicals. WHO. April 2004. https://www.who.int/vaccine_research/documents/en/gvrf2003.pdf. "Epidemiology of Helicobacter pylori and gastric cancer…"
- ↑ "Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis". Cancer Science 96 (12): 835–843. December 2005. doi:10.1111/j.1349-7006.2005.00130.x. PMID 16367902.
- ↑ Usui, Yoshiaki; Taniyama, Yukari; Endo, Mikiko; Koyanagi, Yuriko N.; Kasugai, Yumiko; Oze, Isao; Ito, Hidemi; Imoto, Issei et al. (2023-03-30). "Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer" (in en). New England Journal of Medicine 388 (13): 1181–1190. doi:10.1056/NEJMoa2211807. ISSN 0028-4793. PMID 36988593. http://www.nejm.org/doi/10.1056/NEJMoa2211807.
- ↑ "Developing a vaccine for the Epstein–Barr Virus could prevent up to 200,000 cancers globally say experts". 24 March 2014. http://www.cancerresearchuk.org/about-us/cancer-news/press-release/2014-03-24-developing-a-vaccine-for-the-epstein-barr-virus-could-prevent-up-to-200000-cancers-globally-say.
- ↑ 32.0 32.1 "What Are The Risk Factors For Stomach Cancer(Website)". American Cancer Society. http://www.cancer.org/cancer/stomachcancer/detailedguide/stomach-cancer-risk-factors.
- ↑ "Cigarette smoking and stomach cancer". Cancer Research 50 (21): 7084. November 1990. PMID 2208177. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=2208177.
- ↑ "Tobacco smoking and gastric cancer: review and meta-analysis". International Journal of Cancer 72 (4): 565–573. August 1997. doi:10.1002/(SICI)1097-0215(19970807)72:4<565::AID-IJC3>3.0.CO;2-O. PMID 9259392.
- ↑ 35.0 35.1 35.2 35.3 "The diagnosis and management of gastric cancer". BMJ 347 (16): f6367. November 2013. doi:10.1136/bmj.f6367. PMID 24191271.
- ↑ "Environmental iodine deficiency: A challenge to the evolution of terrestrial life?". Thyroid 10 (8): 727–729. August 2000. doi:10.1089/10507250050137851. PMID 11014322.
- ↑ "Nature, Nurture, and Cancer Risks: Genetic and Nutritional Contributions to Cancer". Annual Review of Nutrition 37: 293–320. August 2017. doi:10.1146/annurev-nutr-071715-051004. PMID 28826375.
- ↑ "Association of Fried Food Intake with Gastric Cancer Risk: A Systemic Review and Meta-Analysis of Case-Control Studies". Nutrients 15 (13): 2982. June 2023. doi:10.3390/nu15132982. PMID 37447308.
- ↑ 39.0 39.1 39.2 "Meat intake and risk of gastric cancer in the Stomach cancer Pooling (StoP) project". Int J Cancer 147 (1): 45–55. July 2020. doi:10.1002/ijc.32707. PMID 31584199.
- ↑ "Human carcinogenesis and bracken fern: a review of the evidence". Current Medicinal Chemistry 9 (6): 675–686. March 2002. doi:10.2174/0929867023370743. PMID 11945131. http://www.benthamdirect.org/pages/content.php?CMC/2002/00000009/00000006/0004C.SGM.
- ↑ "Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence". World Journal of Gastroenterology 12 (27): 4296–4303. July 2006. doi:10.3748/wjg.v12.i27.4296. PMID 16865769.
- ↑ 42.0 42.1 "Fruits and vegetables intake and gastric cancer risk: A pooled analysis within the Stomach cancer Pooling Project". Int J Cancer 147 (11): 3090–3101. December 2020. doi:10.1002/ijc.33134. PMID 32525569.
- ↑ "Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study". The American Journal of Clinical Nutrition 91 (2): 381–390. February 2010. doi:10.3945/ajcn.2009.28209. PMID 20007304.
- ↑ 44.0 44.1 "Epidemiology of gastric cancer". World Journal of Gastroenterology 12 (3): 354–362. January 2006. doi:10.3748/wjg.v12.i3.354. PMID 16489633.
- ↑ "Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications". Annals of Internal Medicine 143 (3): 199–211. August 2005. doi:10.7326/0003-4819-143-3-200508020-00006. PMID 16061918.
- ↑ "22. Sodium Iodide Symporter (NIS) in Gastric Mucosa: Gastric Iodide Secretion". Comprehensive Handbook of Iodine: Nutritional, Biochemical, Pathological and Therapeutic Aspects. Elsevier. 2009. pp. 215–220. ISBN 978-0-12-374135-6.
- ↑ "Evolutionary Significance of Iodine". Current Chemical Biology 5 (3): 155–162. 2011. doi:10.2174/187231311796765012. ISSN 1872-3136.
- ↑ "Role of iodine in evolution and carcinogenesis of thyroid, breast and stomach". Advances in Clinical Pathology 4 (1): 11–17. January 2000. PMID 10936894. http://www.medsci.org/v05p0189.htm.
- ↑ 49.0 49.1 49.2 49.3 49.4 "Hereditary Diffuse Cancer". http://www.nostomachforcancer.org/gastric-cancer/hereditary-diffuse-gastric-cancer.
- ↑ "Association of polymorphisms in TP53 and the promoter region of IL10 with gastric cancer in a Kazakh population". Bosnian Journal of Basic Medical Sciences 20 (4): 539–546. November 2020. doi:10.17305/bjbms.2020.4761. PMID 32651972.
- ↑ "Gastric Cancer — Adenocarcinoma". International Cancer Genome Consortium. http://www.icgc.org/icgc/cgp/69/509/70268.
- ↑ "Gastric Cancer—Intestinal- and diffuse-type". International Cancer Genome Consortium. http://www.icgc.org/icgc/cgp/69/371/811.
- ↑ "Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria". Journal of Medical Genetics 41 (7): 508–517. July 2004. doi:10.1136/jmg.2004.018275. PMID 15235021.
- ↑ "Diabetes and gastric cancer: the potential links". World Journal of Gastroenterology 20 (7): 1701–1711. February 2014. doi:10.3748/wjg.v20.i7.1701. PMID 24587649.
- ↑ "Gastric neuroendocrine tumours". Digestive Surgery 29 (4): 331–348. 2004. doi:10.1159/000342988. PMID 23075625.
- ↑ "Menetrier's disease in korea: report of two cases and review of cases in a gastric cancer prevalent region". Yonsei Medical Journal 45 (3): 555–560. June 2004. doi:10.3349/ymj.2004.45.3.555. PMID 15227748.
- ↑ "Gastric-and-intestinal mixed-type intestinal metaplasia: aberrant expression of transcription factors and stem cell intestinalization". Gastric Cancer 9 (3): 156–166. 2006. doi:10.1007/s10120-006-0375-6. PMID 16952033.
- ↑ "Association of Foxp3 rs3761548 polymorphism with cytokines concentration in gastric adenocarcinoma patients". Cytokine 138: 155351. February 2021. doi:10.1016/j.cyto.2020.155351. PMID 33127257.
- ↑ "The relationship between gastric cancer, its precancerous lesions and bile reflux: A retrospective study". J Dig Dis 21 (4): 222–229. April 2020. doi:10.1111/1751-2980.12858. PMID 32187838.
- ↑ "Gastric Cancer". https://www.lecturio.com/concepts/gastric-cancer/.
- ↑ "Neoplastic stomach lesions and their mimickers: spectrum of imaging manifestations". Cancer Imaging 12 (1): 269–278. August 2012. doi:10.1102/1470-7330.2012.0031. PMID 22935192.
- ↑ "A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions". British Journal of Cancer 108 (4): 941–950. March 2013. doi:10.1038/bjc.2013.44. PMID 23462808.
- ↑ "Breath Test Could Detect And Diagnose Stomach Cancer". Medical News Today. 6 March 2013. http://www.medicalnewstoday.com/articles/257245.php.
- ↑ "Detection of precancerous gastric lesions and gastric cancer through exhaled breath". Gut 65 (3): 400–407. March 2016. doi:10.1136/gutjnl-2014-308536. PMID 25869737.
- ↑ "Volatile organic compounds as a potential screening tool for neoplasm of the digestive system: a meta-analysis". Scientific Reports 11 (1): 23716. December 2021. doi:10.1038/s41598-021-02906-8. PMID 34887450. Bibcode: 2021NatSR..1123716W.
- ↑ "Technology insight: Laser-scanning confocal microscopy and endocytoscopy for cellular observation of the gastrointestinal tract". Nature Clinical Practice. Gastroenterology & Hepatology 2 (1): 31–37. January 2005. doi:10.1038/ncpgasthep0072. PMID 16265098.
- ↑ "Oral acanthosis nigricans, tripe palms and sign of leser-trélat in a patient with gastric adenocarcinoma". International Journal of Dermatology 43 (7): 530–532. July 2004. doi:10.1111/j.1365-4632.2004.02159.x. PMID 15230897.
- ↑ "Occult Gastrointestinal Bleeding: Detection, Interpretation, and Evaluation". JIACM 3 (2): 153–58. 2002. http://medind.nic.in/jac/t02/i2/jact02i2p153.pdf.
- ↑ "Helicobacter pylori infection and the risk of gastric carcinoma". The New England Journal of Medicine 325 (16): 1127–1131. October 1991. doi:10.1056/NEJM199110173251603. PMID 1891020.
- ↑ Pathologic Basis of Disease (8th ed.). Saunders Elsevier. 2010. p. 784. ISBN 978-1-4160-3121-5.
- ↑ Kumar 2010, p. 786
- ↑ "Helicobacter pylori-induced gastric pathology: insights from in vivo and ex vivo models". Disease Models & Mechanisms 10 (2): 89–104. February 2017. doi:10.1242/dmm.027649. PMID 28151409.
- ↑ "Roles of resolvins in the resolution of acute inflammation". Cell Biology International 39 (1): 3–22. January 2015. doi:10.1002/cbin.10345. PMID 25052386.
- ↑ "CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy". Radiographics 26 (1): 143–156. 2006. doi:10.1148/rg.261055078. PMID 16418249.
- ↑ "Detailed Guide: Stomach Cancer Treatment Choices by Type and Stage of Stomach Cancer". American Cancer Society. 2009-11-03. http://www.cancer.org/docroot/cri/content/cri_2_4_4x_treatment_choices_by_type_and_stage_of_stomach_cancer_40.asp.
- ↑ "What Are The Stages Of Stomach Cancer?". ehealthmd.com. October 2009. http://www.ehealthmd.com/library/stomachcancer/STC_stages.html.
- ↑ "Detailed Guide: Stomach Cancer: How Is Stomach Cancer Staged?". American Cancer Society. http://www.cancer.org/docroot/CRI/content/CRI_2_4_3X_How_is_stomach_cancer_staged_40.asp.
- ↑ "Impact of open-access endoscopy on detection of early oesophageal and gastric cancer 1994 - 2003: population-based study". Endoscopy 38 (5): 503–507. May 2006. doi:10.1055/s-2006-925124. PMID 16767587.
- ↑ "Survival trends in patients with gastric and esophageal adenocarcinomas: a population-based study". Mayo Clinic Proceedings 83 (10): 1087–1094. October 2008. doi:10.4065/83.10.1087. PMID 18828967.
- ↑ "Incidence and survival of stomach cancer in a high-risk population of Chile". World Journal of Gastroenterology 15 (15): 1854–1862. April 2009. doi:10.3748/wjg.15.1854. PMID 19370783.
- ↑ "Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials". BMJ 348: g3174. May 2014. doi:10.1136/bmj.g3174. PMID 24846275.
- ↑ "Diet and cancer risk in the Korean population: a meta- analysis". Asian Pacific Journal of Cancer Prevention 15 (19): 8509–8519. 2014. doi:10.7314/apjcp.2014.15.19.8509. PMID 25339056.
- ↑ "Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies". PLOS ONE 9 (12): e116060. 2014. doi:10.1371/journal.pone.0116060. PMID 25549091. Bibcode: 2014PLoSO...9k6060K.
- ↑ "Antioxidant supplements for preventing gastrointestinal cancers". The Cochrane Database of Systematic Reviews (3): CD004183. July 2008. doi:10.1002/14651858.CD004183.pub3. PMID 18677777.
- ↑ "Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases". The Cochrane Database of Systematic Reviews 2012 (3): CD007176. March 2012. doi:10.1002/14651858.CD007176.pub2. PMID 22419320.
- ↑ Aziz, Shahid; Rasheed, Faisal; Zahra, Rabaab; König, Simone (January 2022). "Gastric Cancer Pre-Stage Detection and Early Diagnosis of Gastritis Using Serum Protein Signatures" (in en). Molecules 27 (9): 2857. doi:10.3390/molecules27092857. ISSN 1420-3049. PMID 35566209.
- ↑ "Modern oncological approaches to gastric adenocarcinoma". Gastroenterology Clinics of North America 42 (2): 359–369. June 2013. doi:10.1016/j.gtc.2013.01.011. PMID 23639645.
- ↑ "Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer". World Journal of Gastroenterology 19 (32): 5365–5376. August 2013. doi:10.3748/wjg.v19.i32.5365. PMID 23983442.
- ↑ "Chemoradiation therapy: localized esophageal, gastric, and pancreatic cancer". Surgical Oncology Clinics of North America 22 (3): 511–524. July 2013. doi:10.1016/j.soc.2013.02.005. PMID 23622077.
- ↑ 90.0 90.1 "Critical appraisal of trastuzumab in treatment of advanced stomach cancer". Cancer Management and Research 3: 57–64. March 2011. doi:10.2147/CMAR.S12698. PMID 21556317.
- ↑ "Laparoscopic versus open gastrectomy for gastric cancer". The Cochrane Database of Systematic Reviews 2016 (3): CD011389. March 2016. doi:10.1002/14651858.CD011389.pub2. PMID 27030300.
- ↑ 92.0 92.1 Syn, Nicholas L.; Wee, Ian; Shabbir, Asim; Kim, Guowei; So, Jimmy Bok-Yan (December 2018). "Pouch Versus No Pouch Following Total gastrectomy: Meta-analysis of Randomized and Non-randomized Studies". Annals of Surgery 269 (6): 1041–1053. doi:10.1097/sla.0000000000003082. PMID 30571657.
- ↑ "Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis". BMC Cancer 13 (1): 577. December 2013. doi:10.1186/1471-2407-13-577. PMID 24304886. PMC 4235220. http://www.biomedcentral.com/content/pdf/1471-2407-13-577.pdf.
- ↑ "Chemotherapy for advanced gastric cancer: across the years for a standard of care". Expert Opinion on Pharmacotherapy 8 (6): 797–808. April 2007. doi:10.1517/14656566.8.6.797. PMID 17425475.
- ↑ 95.0 95.1 "HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions". Modern Pathology 26 (6): 816–824. June 2013. doi:10.1038/modpathol.2012.228. PMID 23348899.
- ↑ Gastric Cancer at eMedicine
- ↑ "The stomach cancer proteome – The Human Protein Atlas". https://www.proteinatlas.org/humanpathology/stomach+cancer.
- ↑ "A pathology atlas of the human cancer transcriptome". Science 357 (6352): eaan2507. August 2017. doi:10.1126/science.aan2507. PMID 28818916.
- ↑ "P2X7 receptor as an independent prognostic indicator in gastric cancer". Bosnian Journal of Basic Medical Sciences 20 (2): 188–196. May 2020. doi:10.17305/bjbms.2020.4620. PMID 32070268.
- ↑ 100.0 100.1 (in English) WHO report on cancer: setting priorities, investing wisely and providing care for all.. World Health Organization (WHO). 2020. p. 26. ISBN 978-92-4-000129-9.
- ↑ "Global cancer statistics, 2002". CA: A Cancer Journal for Clinicians 55 (2): 74–108. 2005. doi:10.3322/canjclin.55.2.74. PMID 15761078.
- ↑ "Are the number of cancer cases increasing or decreasing in the world?". WHO Online Q&A. WHO. 1 April 2008. https://www.who.int/features/qa/15/en/index.html.
- ↑ World Cancer Report 2014. 2014.
- ↑ "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet 380 (9859): 2095–2128. December 2012. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604. PMC 10790329. https://repozitorij.upr.si/Dokument.php?id=7123&dn=.
- ↑ "PRESS RELEASE N° 224 Global battle against cancer won't be won with treatment alone: Effective prevention measures urgently needed to prevent cancer crisis". World Health Organization. 3 February 2014. http://www.iarc.fr/en/media-centre/pr/2014/pdfs/pr224_E.pdf.
- ↑ "Câncer gástrico em adultos jovens". Rev. Bras. Cancerol. 46 (3): 299–304. 2000. doi:10.32635/2176-9745.RBC.2000v46n3.2441. http://www.inca.gov.br/rbc/n_46/v03/english/article6.html.
- ↑ "Health profile: United States". Le Duc Media. http://www.worldlifeexpectancy.com/country-health-profile/united-states.
- ↑ "Health profile: China". Le Duc Media. http://www.worldlifeexpectancy.com/country-health-profile/china.
- ↑ "Stomach Cancer: Death Rate Per 100,000". Le Duc Media. http://www.worldlifeexpectancy.com/cause-of-death/stomach-cancer/by-country/.
- ↑ "Stomach cancer statistics". http://www.cancerresearchuk.org/cancer-info/cancerstats/types/stomach/.
- ↑ 111.0 111.1 111.2 111.3 "Gastric cancer in Africa: current management and outcomes". World Journal of Gastroenterology 20 (14): 3875–3879. April 2014. doi:10.3748/wjg.v20.i14.3875. PMID 24833842.
- ↑ 112.0 112.1 "The relationship between Helicobacter pylori infection, the virulence genotypes of the infecting strain and gastric cancer in the African setting". Helicobacter 6 (4): 268–273. December 2001. doi:10.1046/j.1523-5378.2001.00044.x. PMID 11843958.
- ↑ "22 Cancer of the Gastrointestinal tract § E: Gastric Cancer: Diagnostic Techniques and Work-Up". Withrow and MacEwen's Small Animal Clinical Oncology (5th ed.). Elsevier. 2013. pp. 402–3. ISBN 9780323241977. https://books.google.com/books?id=nYYlauGf0D4C&pg=PA402.
- ↑ Alizadeh M, Raufman JP. Gastrointestinal neoplasia: carcinogenic interaction between bile acids and Helicobacter pylori in the stomach. J Clin Invest 2022;132:e160194
- ↑ Noto JM, Piazuelo MB, Shah SC, Romero-Gallo J, Hart JL, Di C, Carmichael JD, Delgado AG, Halvorson AE, Greevy RA, Wroblewski LE, Sharma A, Newton AB, Allaman MM, Wilson KT, Washington MK, Calcutt MW, Schey KL, Cummings BP, Flynn CR, Zackular JP, Peek RM Jr. Iron deficiency linked to altered bile acid metabolism promotes Helicobacter pylori-induced inflammation-driven gastric carcinogenesis. J Clin Invest 2022;132:e14782
External links
| Classification | |
|---|---|
| External resources |
- "Gastric cancer treatment guidelines". National Cancer Institute. 14 October 2022. https://www.cancer.gov/types/stomach/hp/stomach-treatment-pdq#section/all.
 |
 KSF
KSF