Bioluminescence
Topic: Physics
 From HandWiki - Reading time: 33 min
From HandWiki - Reading time: 33 min



File:Bioluminescent beetle Elateroidea (video).webm Bioluminescence is the production and emission of light by living organisms. It is a form of chemiluminescence. Bioluminescence occurs widely in marine vertebrates and invertebrates, as well as in some fungi, microorganisms including some bioluminescent bacteria, and terrestrial arthropods such as fireflies. In some animals, the light is bacteriogenic, produced by symbiotic bacteria such as those from the genus Vibrio;[1] in others, it is autogenic, produced by the animals themselves.
In a general sense, the principal chemical reaction in bioluminescence involves a light-emitting molecule and an enzyme, generally called luciferin and luciferase, respectively. Because these are generic names, luciferins and luciferases are often distinguished by the species or group, e.g. firefly luciferin. In all characterized cases, the enzyme catalyzes the oxidation of the luciferin.
In some species, the luciferase requires other cofactors, such as calcium or magnesium ions, and sometimes also the energy-carrying molecule adenosine triphosphate (ATP). In evolution, luciferins vary little: one in particular, coelenterazine, is found in 11 different animal phyla, though in some of these, the animals obtain it through their diet. Conversely, luciferases vary widely between different species, which is evidence that bioluminescence has arisen over 40 times in evolutionary history.
Both Aristotle and Pliny the Elder mentioned that damp wood sometimes gives off a glow. Many centuries later Robert Boyle showed that oxygen was involved in the process, in both wood and glowworms. It was not until the late nineteenth century that bioluminescence was properly investigated. The phenomenon is widely distributed among animal groups, especially in marine environments. On land it occurs in fungi, bacteria and some groups of invertebrates, including insects.
The uses of bioluminescence by animals include counterillumination camouflage, mimicry of other animals, for example to lure prey, and signaling to other individuals of the same species, such as to attract mates. In the laboratory, luciferase-based systems are used in genetic engineering and biomedical research. Researchers are also investigating the possibility of using bioluminescent systems for street and decorative lighting, and a bioluminescent plant has been created.[2]
History
Before the development of the safety lamp for use in coal mines, dried fish skins were used in Britain and Europe as a weak source of light.[3] This experimental form of illumination avoided the necessity of using candles which risked sparking explosions of firedamp.[4] Another safe source of illumination in mines was bottles containing fireflies.[5] In 1920, the American zoologist E. Newton Harvey published a monograph, The Nature of Animal Light, summarizing early work on bioluminescence. Harvey notes that Aristotle mentions light produced by dead fish and flesh, and that both Aristotle and Pliny the Elder (in his Natural History) mention light from damp wood. He also records that Robert Boyle experimented on these light sources, and showed that both they and the glowworm require air for light to be produced. Harvey notes that in 1753, J. Baker identified the flagellate Noctiluca "as a luminous animal" "just visible to the naked eye",[6] and in 1854 Johann Florian Heller (1813–1871) identified strands (hyphae) of fungi as the source of light in dead wood.[7]
Tuckey, in his posthumous 1818 Narrative of the Expedition to the Zaire, described catching the animals responsible for luminescence. He mentions pellucids, crustaceans (to which he ascribes the milky whiteness of the water), and cancers (shrimps and crabs). Under the microscope he described the "luminous property" to be in the brain, resembling "a most brilliant amethyst about the size of a large pin's head".[8]
Charles Darwin noticed bioluminescence in the sea, describing it in his Journal:
While sailing in these latitudes on one very dark night, the sea presented a wonderful and most beautiful spectacle. There was a fresh breeze, and every part of the surface, which during the day is seen as foam, now glowed with a pale light. The vessel drove before her bows two billows of liquid phosphorus, and in her wake she was followed by a milky train. As far as the eye reached, the crest of every wave was bright, and the sky above the horizon, from the reflected glare of these livid flames, was not so utterly obscure, as over the rest of the heavens.[9]
Darwin also observed a luminous "jelly-fish of the genus Dianaea",[9] noting that: "When the waves scintillate with bright green sparks, I believe it is generally owing to minute crustacea. But there can be no doubt that very many other pelagic animals, when alive, are phosphorescent."[9] He guessed that "a disturbed electrical condition of the atmosphere"[9] was probably responsible. Daniel Pauly comments that Darwin "was lucky with most of his guesses, but not here",[10] noting that biochemistry was too little known, and that the complex evolution of the marine animals involved "would have been too much for comfort".[10]

Bioluminescence attracted the attention of the United States Navy in the Cold War, since submarines in some waters can create a bright enough wake to be detected; a German submarine was sunk in the First World War, having been detected in this way. The navy was interested in predicting when such detection would be possible, and hence guiding their own submarines to avoid detection.[12]
Among the anecdotes of navigation by bioluminescence is one recounted by the Apollo 13 astronaut Jim Lovell, who as a navy pilot had found his way back to his aircraft carrier USS Shangri-La when his navigation systems failed. Turning off his cabin lights, he saw the glowing wake of the ship, and was able to fly to it and land safely.[13]
The France pharmacologist Raphaël Dubois carried out work on bioluminescence in the late nineteenth century. He studied click beetles (Pyrophorus) and the marine bivalve mollusc Pholas dactylus. He refuted the old idea that bioluminescence came from phosphorus,[14][lower-alpha 1] and demonstrated that the process was related to the oxidation of a specific compound, which he named luciferin, by an enzyme.[16] He sent Harvey siphons from the mollusc preserved in sugar. Harvey had become interested in bioluminescence as a result of visiting the South Pacific and Japan and observing phosphorescent organisms there. He studied the phenomenon for many years. His research aimed to demonstrate that luciferin, and the enzymes that act on it to produce light, were interchangeable between species, showing that all bioluminescent organisms had a common ancestor. However, he found this hypothesis to be false, with different organisms having major differences in the composition of their light-producing proteins. He spent the next 30 years purifying and studying the components, but it fell to the young Japanese chemist Osamu Shimomura to be the first to obtain crystalline luciferin. He used the sea firefly Vargula hilgendorfii, but it was another ten years before he discovered the chemical's structure and published his 1957 paper Crystalline Cypridina Luciferin.[17] Shimomura, Martin Chalfie, and Roger Y. Tsien won the 2008 Nobel Prize in Chemistry for their 1961 discovery and development of green fluorescent protein as a tool for biological research.[18]
Harvey wrote a detailed historical account on all forms of luminescence in 1957.[19] An updated book on bioluminescence covering also the twentieth and early twenty-first century was published recently.[20][21]
Evolution
In 1932 E. N. Harvey was among the first to propose how bioluminescence could have evolved.[22] In this early paper, he suggested that proto-bioluminescence could have arisen from respiratory chain proteins that hold fluorescent groups. This hypothesis has since been disproven, but it did lead to considerable interest in the origins of the phenomenon. Today, the two prevailing hypotheses (both concerning marine bioluminescence) are those put forth by Howard Seliger in 1993 and Rees et al. in 1998.[23][24]
Seliger's theory identifies luciferase enzymes as the catalyst for the evolution of bioluminescent systems. It suggests that the original purpose of luciferases was as mixed-function oxygenases. As the early ancestors of many species moved into deeper and darker waters natural selection favored the development of increased eye sensitivity and enhanced visual signals.[25] If selection were to favor a mutation in the oxygenase enzyme required for the breakdown of pigment molecules (molecules often associated with spots used to attract a mate or distract a predator) it could have eventually resulted in external luminescence in tissues.[23]
Rees et al. use evidence gathered from the marine luciferin coelenterazine to suggest that selection acting on luciferins may have arisen from pressures to protect oceanic organisms from potentially deleterious reactive oxygen species (e.g. H2O2 and O2− ). The functional shift from antioxidation to bioluminescence probably occurred when the strength of selection for antioxidation defense decreased as early species moved further down the water column. At greater depths exposure to ROS is significantly lower, as is the endogenous production of ROS through metabolism.[24]
While popular at first, Seliger's theory has been challenged, particularly on the biochemical and genetic evidence that Rees examines. What remains clear, however, is that bioluminescence has evolved independently at least 40 times.[26] Bioluminescence in fish began at least by the Cretaceous period. About 1,500 fish species are known to be bioluminescent; the capability evolved independently at least 27 times. Of these, 17 involved the taking up of bioluminous bacteria from the surrounding water while in the others, the intrinsic light evolved through chemical synthesis. These fish have become surprisingly diverse in the deep ocean and control their light with the help of their nervous system, using it not just to lure prey or hide from predators, but also for communication.[27][28]
All bioluminescent organisms have in common that the reaction of a "luciferin" and oxygen is catalyzed by a luciferase to produce light.[29] McElroy and Seliger proposed in 1962 that the bioluminescent reaction evolved to detoxify oxygen, in parallel with photosynthesis.[30]
Thuesen, Davis et al. showed in 2016 that bioluminescence has evolved independently 27 times within 14 fish clades across ray-finned fishes.[27] The oldest of these appears to be Stomiiformes and Myctophidae.[31] In sharks, bioluminiscence has evolved only once.[32]
Chemical mechanism

Bioluminescence is a form of chemiluminescence where light energy is released by a chemical reaction. This reaction involves a light-emitting pigment, the luciferin, and a luciferase, the enzyme component.[33] Because of the diversity of luciferin/luciferase combinations, there are very few commonalities in the chemical mechanism. From currently studied systems, the only unifying mechanism is the role of molecular oxygen; often there is a concurrent release of carbon dioxide (CO2). For example, the firefly luciferin/luciferase reaction requires magnesium and ATP and produces CO2, adenosine monophosphate (AMP) and pyrophosphate (PP) as waste products. Other cofactors may be required, such as calcium (Ca2+) for the photoprotein aequorin, or magnesium (Mg2+) ions and ATP for the firefly luciferase.[34] Generically, this reaction can be described as:
- Luciferin + O2Oxyluciferin + light energy

Instead of a luciferase, the jellyfish Aequorea victoria makes use of another type of protein called a photoprotein, in this case specifically aequorin.[35] When calcium ions are added, rapid catalysis creates a brief flash quite unlike the prolonged glow produced by luciferase. In a second, much slower step, luciferin is regenerated from the oxidized (oxyluciferin) form, allowing it to recombine with aequorin, in preparation for a subsequent flash. Photoproteins are thus enzymes, but with unusual reaction kinetics.[36] Furthermore, some of the blue light released by aequorin in contact with calcium ions is absorbed by a green fluorescent protein, which in turn releases green light in a process called resonant energy transfer.[37]
Overall, bioluminescence has arisen over 40 times in evolutionary history.[33] In evolution, luciferins tend to vary little: one in particular, coelenterazine, is the light emitting pigment for nine phyla (groups of very different organisms), including polycystine radiolaria, Cercozoa (Phaeodaria), protozoa, comb jellies, cnidaria including jellyfish and corals, crustaceans, molluscs, arrow worms and vertebrates (ray-finned fish). Not all these organisms synthesise coelenterazine: some of them obtain it through their diet.[33] Conversely, luciferase enzymes vary widely and tend to be different in each species.[33]
Distribution
File:Surfing on bioluminescent wave.webm
Bioluminescence occurs widely among animals, especially in the open sea, including fish, jellyfish, comb jellies, crustaceans, and cephalopod molluscs; in some fungi and bacteria; and in various terrestrial invertebrates including insects. In marine coastal habitats, about 2.5% of organisms are estimated to be bioluminescent, whereas in pelagic habitats in the eastern Pacific, about 76% of the main taxa of deep-sea animals have been found to be capable of producing light.[38] More than 700 animal genera have been recorded with light-producing species.[39] Most marine light-emission is in the blue and green light spectrum. However, some loose-jawed fish emit red and infrared light, and the genus Tomopteris emits yellow light.[33][40]
The most frequently encountered bioluminescent organisms may be the dinoflagellates in the surface layers of the sea, which are responsible for the sparkling luminescence sometimes seen at night in disturbed water. At least 18 genera exhibit luminosity.[33] Luminescent dinoflagellate ecosystems are present in warm water lagoons and bays with narrow openings to the ocean.[41] A different effect is the thousands of square miles of the ocean which shine with the light produced by bioluminescent bacteria, known as mareel or the milky seas effect.[42]
Pelagic zone
Bioluminescence is abundant in the pelagic zone, with the most concentration at depths devoid of light and surface waters at night. These organisms participate in diurnal vertical migration from the dark depths to the surface at night, dispersing the population of bioluminescent organisms across the pelagic water column. The dispersal of bioluminescence across different depths in the pelagic zone has been attributed to the selection pressures imposed by predation and the lack of places to hide in the open sea. In depths where sunlight never penetrates, often below 200m, the significance of bioluminescent is evident in the retainment of functional eyes for organisms to detect bioluminescence.[43]
Bacterial symbioses
Organisms often produce bioluminescence themselves, rarely do they generate it from outside phenomena. However, there are occasions where bioluminescence is produced by bacterial symbionts that have a symbiotic relationship with the host organism. Although many luminous bacteria in the marine environment are free-living, a majority are found in symbiotic relationships that involve fish, squids, crustaceans etc. as hosts. Most luminous bacterial inhabit the marine sea, with Photobacterium and Vibrio genera dominating the marine environment.[44]
In the symbiotic relationship, bacterium benefit from having a source of nourishment and a refuge to grow. Hosts obtain these bacterial symbionts either from the environment, spawning, or the luminous bacterium is evolving with their host.[45] Coevolutionary interactions are suggested as host organisms’ anatomical adaptations have become specific to only certain luminous bacteria, to suffice ecological dependence of bioluminescence.[46]
Benthic zone
Bioluminescence is widely studied amongst species located in the mesopelagic zone, but the benthic zone at mesopelagic depths has remained widely unknown. Benthic habitats at depths beyond the mesopelagic are also poorly understood due to the same constraints. Unlike the pelagic zone where the emission of light is undisturbed in the open sea, the occurrence of bioluminescence in the benthic zone is less common. It has been attributed to the blockage of emitted light by a number of sources such as the sea floor, and inorganic and organic structures. Visual signals and communication that is prevalent in the pelagic zone such as counterillumination may not be functional or relevant in the benthic realm. Bioluminescence in bathyal benthic species still remains poorly studied due to difficulties of the collection of species at these depths.[47]
Uses in nature

Bioluminescence has several functions in different taxa. Steven Haddock et al. (2010) list as more or less definite functions in marine organisms the following: defensive functions of startle, counterillumination (camouflage), misdirection (smoke screen), distractive body parts, burglar alarm (making predators easier for higher predators to see), and warning to deter settlers; offensive functions of lure, stun or confuse prey, illuminate prey, and mate attraction/recognition. It is much easier for researchers to detect that a species is able to produce light than to analyze the chemical mechanisms or to prove what function the light serves.[33] In some cases the function is unknown, as with species in three families of earthworm (Oligochaeta), such as Diplocardia longa where the coelomic fluid produces light when the animal moves.[48] The following functions are reasonably well established in the named organisms.
Counterillumination camouflage
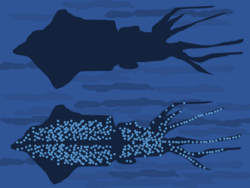
In many animals of the deep sea, including several squid species, bacterial bioluminescence is used for camouflage by counterillumination, in which the animal matches the overhead environmental light as seen from below.[49] In these animals, photoreceptors control the illumination to match the brightness of the background.[49] These light organs are usually separate from the tissue containing the bioluminescent bacteria. However, in one species, Euprymna scolopes, the bacteria are an integral component of the animal's light organ.[50]
Attraction

Bioluminescence is used in a variety of ways and for different purposes. The cirrate octopod Stauroteuthis syrtensis uses emits bioluminescence from its sucker like structures.[51] These structures are believed to have evolved from what are more commonly known as octopus suckers. They do not have the same function as the normal suckers because they no longer have any handling or grappling ability due its evolution of photophores. The placement of the photophores are within the animals oral reach, which leads researchers to suggest that it uses it bioluminescence to capture and lure prey.[52]
Fireflies use light to attract mates. Two systems are involved according to species; in one, females emit light from their abdomens to attract males; in the other, flying males emit signals to which the sometimes sedentary females respond.[48][53] Click beetles emit an orange light from the abdomen when flying and a green light from the thorax when they are disturbed or moving about on the ground. The former is probably a sexual attractant but the latter may be defensive.[48] Larvae of the click beetle Pyrophorus nyctophanus live in the surface layers of termite mounds in Brazil. They light up the mounds by emitting a bright greenish glow which attracts the flying insects on which they feed.[48]
In the marine environment, use of luminescence for mate attraction is chiefly known among ostracods, small shrimplike crustaceans, especially in the family Cyprididae. Pheromones may be used for long-distance communication, with bioluminescence used at close range to enable mates to "home in".[33] A polychaete worm, the Bermuda fireworm creates a brief display, a few nights after the full moon, when the female lights up to attract males.[54]
Defense

The defense mechanisms for bioluminescent organisms can come in multiple forms; startling prey, counterillumination, smoke screen or misdirection, distractive body parts, burglar alarm, sacrificial tag or warning coloration. The shrimp family Oplophoridae Dana use their bioluminescence as a way of startling the predator that is after them.[55] Acanthephyra purpurea, within the Oplophoridae family, uses its photophores to emit light, and can secrete a bioluminescent substance when in the presence of a predator. This secretory mechanism is common among prey fish.[55]
Many cephalopods, including at least 70 genera of squid, are bioluminescent.[33] Some squid and small crustaceans use bioluminescent chemical mixtures or bacterial slurries in the same way as many squid use ink. A cloud of luminescent material is expelled, distracting or repelling a potential predator, while the animal escapes to safety.[33] The deep sea squid Octopoteuthis deletron may autotomise portions of its arms which are luminous and continue to twitch and flash, thus distracting a predator while the animal flees.[33]
Dinoflagellates may use bioluminescence for defense against predators. They shine when they detect a predator, possibly making the predator itself more vulnerable by attracting the attention of predators from higher trophic levels.[33] Grazing copepods release any phytoplankton cells that flash, unharmed; if they were eaten they would make the copepods glow, attracting predators, so the phytoplankton's bioluminescence is defensive. The problem of shining stomach contents is solved (and the explanation corroborated) in predatory deep-sea fishes: their stomachs have a black lining able to keep the light from any bioluminescent fish prey which they have swallowed from attracting larger predators.[10]
The sea-firefly is a small crustacean living in sediment. At rest it emits a dull glow but when disturbed it darts away leaving a cloud of shimmering blue light to confuse the predator. During World War II it was gathered and dried for use by the Japanese army as a source of light during clandestine operations.[17]
The larvae of railroad worms (Phrixothrix) have paired photic organs on each body segment, able to glow with green light; these are thought to have a defensive purpose.[56] They also have organs on the head which produce red light; they are the only terrestrial organisms to emit light of this color.[57]
Warning
Aposematism is a widely used function of bioluminescence, providing a warning that the creature concerned is unpalatable. It is suggested that many firefly larvae glow to repel predators; some millipedes glow for the same purpose.[58] Some marine organisms are believed to emit light for a similar reason. These include scale worms, jellyfish and brittle stars but further research is needed to fully establish the function of the luminescence. Such a mechanism would be of particular advantage to soft-bodied cnidarians if they were able to deter predation in this way.[33] The limpet Latia neritoides is the only known freshwater gastropod that emits light. It produces greenish luminescent mucus which may have an anti-predator function.[59] The marine snail Hinea brasiliana uses flashes of light, probably to deter predators. The blue-green light is emitted through the translucent shell, which functions as an efficient diffuser of light.[60]
Communication
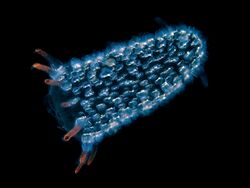
Communication in the form of quorum sensing plays a role in the regulation of luminescence in many species of bacteria. Small extracellularly secreted molecules stimulate the bacteria to turn on genes for light production when cell density, measured by concentration of the secreted molecules, is high.[33]
Pyrosomes are colonial tunicates and each zooid has a pair of luminescent organs on either side of the inlet siphon. When stimulated by light, these turn on and off, causing rhythmic flashing. No neural pathway runs between the zooids, but each responds to the light produced by other individuals, and even to light from other nearby colonies.[61] Communication by light emission between the zooids enables coordination of colony effort, for example in swimming where each zooid provides part of the propulsive force.[62]
Some bioluminous bacteria infect nematodes that parasitize Lepidoptera larvae. When these caterpillars die, their luminosity may attract predators to the dead insect thus assisting in the dispersal of both bacteria and nematodes.[48] A similar reason may account for the many species of fungi that emit light. Species in the genera Armillaria, Mycena, Omphalotus, Panellus, Pleurotus and others do this, emitting usually greenish light from the mycelium, cap and gills. This may attract night-flying insects and aid in spore dispersal, but other functions may also be involved.[48]
Quantula striata is the only known bioluminescent terrestrial mollusc. Pulses of light are emitted from a gland near the front of the foot and may have a communicative function, although the adaptive significance is not fully understood.[63]
Mimicry

Bioluminescence is used by a variety of animals to mimic other species. Many species of deep sea fish such as the anglerfish and dragonfish make use of aggressive mimicry to attract prey. They have an appendage on their heads called an esca that contains bioluminescent bacteria able to produce a long-lasting glow which the fish can control. The glowing esca is dangled or waved about to lure small animals to within striking distance of the fish.[33][64]
The cookiecutter shark uses bioluminescence to camouflage its underside by counterillumination, but a small patch near its pectoral fins remains dark, appearing as a small fish to large predatory fish like tuna and mackerel swimming beneath it. When such fish approach the lure, they are bitten by the shark.[65][66]
Female Photuris fireflies sometimes mimic the light pattern of another firefly, Photinus, to attract its males as prey. In this way they obtain both food and the defensive chemicals named lucibufagins, which Photuris cannot synthesize.[67]
South American giant cockroaches of the genus Lucihormetica were believed to be the first known example of defensive mimicry, emitting light in imitation of bioluminescent, poisonous click beetles.[68] However, doubt has been cast on this assertion, and there is no conclusive evidence that the cockroaches are bioluminescent.[69][70]

Illumination
While most marine bioluminescence is green to blue, some deep sea barbeled dragonfishes in the genera Aristostomias, Pachystomias and Malacosteus emit a red glow. This adaptation allows the fish to see red-pigmented prey, which are normally invisible to other organisms in the deep ocean environment where red light has been filtered out by the water column.[71] The fish is able to utilize the longer wavelength to act as a spotlight for its prey that only it is able to see.[71] In addition to the utilization of the light for predation, it has been hypothesized that the fish use this to communicate with each other to find potential mates.[72] The ability of the fish to see this light is explained by the presence of specialized rhodopsin pigment.[71] The angler siphonophore (Erenna), also utilizes red bioluminescence in appendages to lure fish.[73]
The mechanism of light creation is through a suborbital photophore that utilizes gland cells which produce exergonic chemical reactions that produce light with a longer, red wavelength.[73] The dragonfish species which produce the red light also produce blue light in photophore on the dorsal area.[73] The main function of this is to alert the fish to the presence of its prey.[74] The additional pigment is thought to be assimilated from chlorophyll derivatives found in the copepods which form part of its diet.[74]
Biotechnology
Biology and medicine
Bioluminescent organisms are a target for many areas of research. Luciferase systems are widely used in genetic engineering as reporter genes, each producing a different color by fluorescence,[75][76] and for biomedical research using bioluminescence imaging.[77][78][79] For example, the firefly luciferase gene was used as early as 1986 for research using transgenic tobacco plants.[80] Vibrio bacteria symbiose with marine invertebrates such as the Hawaiian bobtail squid (Euprymna scolopes), are key experimental models for bioluminescence.[81][82] Bioluminescent activated destruction is an experimental cancer treatment.[83]
In Vivo luminescence cell and animal imaging uses dyes and fluorescent proteins as chromophores. The characteristics of each chromophore determine which cell area(s) will be targeted and illuminated.[84]
Light production
The structures of photophores, the light producing organs in bioluminescent organisms, are being investigated by industrial designers. Engineered bioluminescence could perhaps one day be used to reduce the need for street lighting, or for decorative purposes if it becomes possible to produce light that is both bright enough and can be sustained for long periods at a workable price.[12][85][86] The gene that makes the tails of fireflies glow has been added to mustard plants. The plants glow faintly for an hour when touched, but a sensitive camera is needed to see the glow.[87] University of Wisconsin–Madison is researching the use of genetically engineered bioluminescent E. coli bacteria, for use as bioluminescent bacteria in a light bulb.[88] In 2011, Philips launched a microbial system for ambience lighting in the home.[89][90] An iGEM team from Cambridge (England) has started to address the problem that luciferin is consumed in the light-producing reaction by developing a genetic biotechnology part that codes for a luciferin regenerating enzyme from the North American firefly.[91] In 2016, Glowee, a French company started selling bioluminescent lights for shop fronts and street signs,[92] for use between 1 and 7 in the morning when the law forbids use of electricity for this purpose.[93][94] They used the bioluminescent bacterium Aliivibrio fischeri, but the maximum lifetime of their product was three days.[92] In April 2020, plants were genetically engineered to glow more brightly using genes from the bioluminescent mushroom Neonothopanus nambi to convert caffeic acid into luciferin.[94][95]
ATP bioluminescence
ATP bioluminescence is the process in which ATP is used to generate luminescence in an organism. It proves to be a very good biosensor to test cell viability. Optical biosensors include process of measurement of luminescence, fluorescence absorbance or emission. Through these measurements, quantitative measurement of ATP bioluminescence is applied to detect existence of living microbes only. Since the method is quick and convenient, it results in real-time data. It is faster, economical and easier to work with. Optical biosensors sense the observed optical signal based on measuring the photons involved in the phenomenon (spiking) It depends on the interaction of microbes with analytes. Thus, it is correlated with the concentration of the microbial population which is determined through this method.[96]
Differentiation between living and non living cells
In ATP bioluminescence, it is assumed that all living cells in the same have the same amount of ATP over time during the chemical reaction between luciferin, luciferase to produce ATP, This is done in order to measure the viability of the cell and allows the researcher to measure the amount of living and dead cells in the sample on basis of presence or absence of ATP. Living cells that contain ATP produce a bioluminescent flash due to the luciferin-luciferase reaction in presence of ATP. Dead cells do not produce any bioluminescence due to absence of ATP. The amount of the intensity of the signal is constant for each living cell in a healthy sample. In this way, the overall number of living cells within a sample is determined.[97]
Process of measurement of microbial population
ATP, which is a fundamental compound in the luciferase reaction, is utilized and in the second step, oxyluciferin is produced. The oxyluciferin is produced in an excited state, which produces light when it goes back to ground state. The light emitted is detected by a luminometer. Concentration of the ATP is directly proportional to the expressed light measured as Relative Light Units (RLU). A receiver operating characteristic (ROC) is used to calculate the sensitivity and specificity of the measurements. There is direct correlation between luminescence intensity and concentration of standard ATP. There is a direct correlation between bioluminescence and colony forming unit (CFU). Thus, concentration of standard ATP and CFU gives a standard correlation. In this way, ATP is measured and microbial population is determined through bioluminescence.[96]
However, it is important to keep in mind that different types of microbial populations are determined through different sets of ATP assays using other substrates and reagents. Renilla and Gaussia based cell viability assays use the substrate coelenterazine.[98]
See also
- Animal coloration
- Biophoton
- Life That Glows, 2016 full-length documentary
Notes
References
- ↑ Ples, Marek (2021-11-11). "Lab Snapshots by Marek Ples; Microbiology - The biology on a different level". https://weirdscience.eu/Mikrobio/?id=1.
- ↑ Callaway, E. 2013. Glowing plants spark debate. Nature, 498:15–16, 4 June 2013. http://www.nature.com/news/glowing-plants-spark-debate-1.13131
- ↑ Smiles, Samuel (1862). Lives of the Engineers. III (George and Robert Stephenson). London: John Murray. p. 107. ISBN 978-0-7153-4281-7. (ISBN refers to the David & Charles reprint of 1968 with an introduction by L. T. C. Rolt)
- ↑ Freese, Barbara (2006). Coal: A Human History. Arrow. p. 51. ISBN 978-0-09-947884-3. https://books.google.com/books?id=p4z7QjaBrCkC&pg=PA51.
- ↑ Fordyce, William (1973). A history of coal, coke and coal fields and the manufacture of iron in the North of England. Graham. ISBN 9780902833999. https://books.google.com/books?id=dlM_AQAAIAAJ.
- ↑ Harvey cites this as Baker, J.: 1743–1753, The Microscope Made Easy and Employment for the Microscope.
- ↑ Harvey, E. Newton (1920). The Nature of Animal Light. Philadelphia & London: J. B. Lippencott. p. 1. https://www.biodiversitylibrary.org/bibliography/57246#/summary.
- ↑ Tuckey, James Hingston (May 1818). Thomson, Thomas. ed. "Narrative of the Expedition to the Zaire". Annals of Philosophy 11 (65): 392. https://www.biodiversitylibrary.org/item/53908#page/412/mode/1up.
- ↑ 9.0 9.1 9.2 9.3 Darwin, Charles (1839). Narrative of the surveying voyages of His Majesty's Ships Adventure and Beagle between the years 1826 and 1836, describing their examination of the southern shores of South America, and the Beagle's circumnavigation of the globe. Journal and remarks. 1832–1836.. Henry Colburn. pp. 190–192. http://darwin-online.org.uk/content/frameset?viewtype=side&itemID=F10.3&pageseq=30.
- ↑ 10.0 10.1 10.2 Pauly, Daniel (13 May 2004). Darwin's Fishes: An Encyclopedia of Ichthyology, Ecology, and Evolution. Cambridge University Press. pp. 15–16. ISBN 978-1-139-45181-9. https://books.google.com/books?id=lTDa0fM4vQIC&pg=PA16.
- ↑ Shimomura, O. (August 1995). "A short story of aequorin". The Biological Bulletin 189 (1): 1–5. doi:10.2307/1542194. PMID 7654844. http://www.biolbull.org/cgi/pmidlookup?view=long&pmid=7654844.
- ↑ 12.0 12.1 "How illuminating". The Economist. 10 March 2011. http://www.economist.com/node/18304146.
- ↑ Huth, John Edward (15 May 2013). The Lost Art of Finding Our Way. Harvard University Press. p. 423. ISBN 978-0-674-07282-4. https://books.google.com/books?id=daT6AAAAQBAJ&pg=PA423.
- ↑ Reshetiloff, Kathy (1 July 2001). "Chesapeake Bay night-lights add sparkle to woods, water". Bay Journal. http://www.bayjournal.com/article/chesapeake_bay_nightlights_add_sparkle_to_woods_water.
- ↑ "Luminescence". Encyclopædia Britannica. https://www.britannica.com/EBchecked/topic/351229/luminescence/68942/Early-investigations.
- ↑ Poisson, Jacques (April 2010). "Raphaël Dubois, from pharmacy to bioluminescence" (in fr). Rev Hist Pharm (Paris) 58 (365): 51–56. doi:10.3406/pharm.2010.22136. ISSN 0035-2349. PMID 20533808.
- ↑ 17.0 17.1 Pieribone, Vincent; Gruber, David F. (2005). Aglow in the Dark: The Revolutionary Science of Biofluorescence. Harvard University Press. pp. 35–41. ISBN 978-0-674-01921-8. https://archive.org/details/aglowindarkrevol00pier.
- ↑ "The Nobel Prize in Chemistry 2008". 8 October 2008. http://nobelprize.org/nobel_prizes/chemistry/laureates/2008/.
- ↑ Harvey, E. Newton (1957). A History of Luminescence: From the Earliest Times Until 1900. Philadelphia: American Philosophical Society.
- ↑ Anctil, Michel (2018). Luminous Creatures: The History and Science of Light Production in Living Organisms. Montreal & Kingston, London, Chicago: McGill-Queen's University Press. ISBN 978-0-7735-5312-5.
- ↑ Fulcher, Bob. "Lovely and Dangerous Lights". Tennessee Conservationist Magazine. http://www.tennessee.gov/environment/conservationist/docs/bluelights.pdf.
- ↑ Harvey, E.N. (1932). "The evolution of bioluminescence and its relation to cell respiration". Proceedings of the American Philosophical Society 71: 135–141.
- ↑ 23.0 23.1 Seliger, H.H. (1993). "Bioluminescence: excited states under cover of darkness". Naval Research Reviews 45.
- ↑ 24.0 24.1 Rees, J. F. (1998). "The origins of marine bioluminescence: Turning oxygen defence mechanisms into deep-sea communication tools". Journal of Experimental Biology 201 (Pt 8): 1211–1221. doi:10.1242/jeb.201.8.1211. PMID 9510532.
- ↑ Widder, Edith A. (1999). Archer, S.. ed. Bioluminescence. Adaptive Mechanisms in the Ecology of Vision. Springer. pp. 555–581.
- ↑ Haddock, S. H. D. (2010). "Bioluminescence in the Sea". Annual Review of Marine Science 2: 443–493. doi:10.1146/annurev-marine-120308-081028. PMID 21141672. Bibcode: 2010ARMS....2..443H.
- ↑ 27.0 27.1 Thuesen, Erik V.; Davis, Matthew P.; Sparks, John S.; Smith, W. Leo (2016). "Repeated and Widespread Evolution of Bioluminescence in Marine Fishes". PLOS ONE 11 (6): e0155154. doi:10.1371/journal.pone.0155154. ISSN 1932-6203. PMID 27276229. Bibcode: 2016PLoSO..1155154D.
- ↑ Yong, Ed (8 June 2016). "Surprising History of Glowing Fish". National Geographic. http://phenomena.nationalgeographic.com/2016/06/08/the-many-origins-of-glowing-fish/.
- ↑ Wilson, Thérèse; Hastings, J. Woodland (1998). "Bioluminescence". Annual Review of Cell and Developmental Biology 14 (1): 197–230. doi:10.1146/annurev.cellbio.14.1.197. PMID 9891783.
- ↑ McElroy, William D.; Seliger, Howard H. (December 1962). "Biological Luminescence". Scientific American 207 (6): 76–91. doi:10.1038/scientificamerican1262-76. ISSN 0036-8733. Bibcode: 1962SciAm.207f..76M.
- ↑ [1]
- ↑ Illuminating the evolution of bioluminescence in sharks
- ↑ 33.00 33.01 33.02 33.03 33.04 33.05 33.06 33.07 33.08 33.09 33.10 33.11 33.12 33.13 33.14 Haddock, Steven H.D.; Moline, Mark A.; Case, James F. (2010). "Bioluminescence in the Sea". Annual Review of Marine Science 2: 443–493. doi:10.1146/annurev-marine-120308-081028. PMID 21141672. Bibcode: 2010ARMS....2..443H.
- ↑ Hastings, J. W. (1983). "Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems". Journal of Molecular Evolution 19 (5): 309–21. doi:10.1007/BF02101634. ISSN 1432-1432. PMID 6358519. Bibcode: 1983JMolE..19..309H.
- ↑ Shimomura, O.; Johnson, F. H.; Saiga, Y. (1962). "Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea". J Cell Comp Physiol 59 (3): 223–39. doi:10.1002/jcp.1030590302. PMID 13911999.
- ↑ Shimomura, O.; Johnson, F.H. (1975). "Regeneration of the photoprotein aequorin". Nature 256 (5514): 236–238. doi:10.1038/256236a0. PMID 239351. Bibcode: 1975Natur.256..236S.
- ↑ Morise, H.; Shimomura, O.; Johnson, F.H.; Winant, J. (1974). "Intermolecular energy transfer in the bioluminescent system of Aequorea". Biochemistry 13 (12): 2656–2662. doi:10.1021/bi00709a028. PMID 4151620.
- ↑ Martini, Séverine; Haddock, Steven H. D. (April 2017). "Quantification of bioluminescence from the surface to the deep sea demonstrates its predominance as an ecological trait". Scientific Reports 7: 45750. doi:10.1038/srep45750. PMID 28374789. Bibcode: 2017NatSR...745750M.
- ↑ Kanie, Shusei; Miura, Daisuke; Jimi, Naoto; Hayashi, Taro; Nakamura, Koji; Sakata, Masahiko; Ogoh, Katsunori; Ohmiya, Yoshihiro et al. (2021-09-27). "Violet bioluminescent Polycirrus sp. (Annelida: Terebelliformia) discovered in the shallow coastal waters of the Noto Peninsula in Japan" (in en). Scientific Reports 11 (1): 19097. doi:10.1038/s41598-021-98105-6. ISSN 2045-2322. PMID 34580316. Bibcode: 2021NatSR..1119097K.
- ↑ Sparks, John S.; Schelly, Robert C.; Smith, W. Leo; Davis, Matthew P.; Tchernov, Dan; Pieribone, Vincent A.; Gruber, David F. (8 January 2014). "The Covert World of Fish Biofluorescence: A Phylogenetically Widespread and Phenotypically Variable Phenomenon". PLOS ONE 9 (1): e83259. doi:10.1371/journal.pone.0083259. PMID 24421880. Bibcode: 2014PLoSO...983259S.
- ↑ "Bioluminescence | National Geographic Society". https://education.nationalgeographic.org/resource/bioluminescence/.
- ↑ Ross, Alison (27 September 2005). "'Milky seas' detected from space". BBC. http://news.bbc.co.uk/1/hi/sci/tech/3760124.stm.
- ↑ Widder, Edith (January 2002). "Bioluminescence and the Pelagic Visual Environment". Marine and Freshwater Behaviour and Physiology 35 (1–2): 1–26. doi:10.1080/10236240290025581. ISSN 1023-6244. http://dx.doi.org/10.1080/10236240290025581.
- ↑ Miyamoto, C.; Skouris, N.; Hosseinkhani, S; Lin, L. Y.; Meighen, E. A. (November 2002). "Common Features of the Quorum Sensing Systems in Vibrio Species". Bioluminescence and Chemiluminescence (World Scientific): 97–100. doi:10.1142/9789812776624_0021. ISBN 978-981-238-156-9. http://dx.doi.org/10.1142/9789812776624_0021.
- ↑ Baker, Lydia J.; Freed, Lindsay L.; Easson, Cole G; Lopez, Jose V; Fenolio, Danté; Sutton, Tracey T.; Nyholm, Spencer V.; Hendry, Tory A (2019-10-01). "Diverse deep-sea anglerfishes share a genetically reduced luminous symbiont that is acquired from the environment" (in en). eLife 8: e47606. doi:10.7554/eLife.47606. ISSN 2050-084X. PMID 31571583.
- ↑ Dunlap, Paul V.; Ast, Jennifer C.; Kimura, Seishi; Fukui, Atsushi; Yoshino, Tetsuo; Endo, Hiromitsu (October 2007). "Phylogenetic analysis of host?symbiont specificity and codivergence in bioluminescent symbioses". Cladistics 23 (5): 507–532. doi:10.1111/j.1096-0031.2007.00157.x. ISSN 0748-3007. http://dx.doi.org/10.1111/j.1096-0031.2007.00157.x.
- ↑ Johnsen, S.; Frank, T. M.; Haddock, S. H. D.; Widder, E. A.; Messing, C. G. (September 2012). "Light and vision in the deep-sea benthos: I. Bioluminescence at 500-1000 m depth in the Bahamian Islands". Journal of Experimental Biology 215 (19): 3335–3343. doi:10.1242/jeb.072009. ISSN 0022-0949. PMID 22956246.
- ↑ 48.0 48.1 48.2 48.3 48.4 48.5 Viviani, Vadim (17 February 2009). "Terrestrial bioluminescence". http://www.photobiology.info/Viviani.html.
- ↑ 49.0 49.1 Young, R. E.; Roper, C. F. (1976). "Bioluminescent countershading in midwater animals: evidence from living squid". Science 191 (4231): 1046–8. doi:10.1126/science.1251214. PMID 1251214. Bibcode: 1976Sci...191.1046Y.
- ↑ Tong, D.; Rozas, N. S.; Oakley, T. H.; Mitchell, J.; Colley, N. J.; McFall-Ngai, M.J. (2009). "Evidence for light perception in a bioluminescent organ". Proceedings of the National Academy of Sciences of the United States of America 106 (24): 9836–41. doi:10.1073/pnas.0904571106. PMID 19509343. Bibcode: 2009PNAS..106.9836T.
- ↑ Johnsen, S.; Balser, E. J.; Fisher, E. C.; Widder, E. A. (1999-08-01). "Bioluminescence in the Deep-Sea Cirrate Octopod Stauroteuthis syrtensis Verrill (Mollusca: Cephalopoda)". The Biological Bulletin 197 (1): 26–39. doi:10.2307/1542994. ISSN 0006-3185. PMID 28296499. https://www.journals.uchicago.edu/doi/10.2307/1542994.
- ↑ Haddock, Steven H.D.; Moline, Mark A.; Case, James F. (2009-12-14). "Bioluminescence in the Sea". Annual Review of Marine Science 2 (1): 443–493. doi:10.1146/annurev-marine-120308-081028. ISSN 1941-1405. PMID 21141672. Bibcode: 2010ARMS....2..443H. https://www.annualreviews.org/doi/10.1146/annurev-marine-120308-081028.
- ↑ Stanger-Hall, K. F.; Lloyd, J.E.; Hillis, D.M. (2007). "Phylogeny of North American fireflies (Coleoptera: Lampyridae): implications for the evolution of light signals". Molecular Phylogenetics and Evolution 45 (1): 33–49. doi:10.1016/j.ympev.2007.05.013. PMID 17644427.
- ↑ Shimomura, Osamu (2012). Bioluminescence: Chemical Principles and Methods. World Scientific. p. 234. ISBN 978-981-4366-08-3. https://books.google.com/books?id=pGyto9ctbVoC&pg=PA234.
- ↑ 55.0 55.1 Wong, Juliet M.; Pérez-Moreno, Jorge L.; Chan, Tin-Yam; Frank, Tamara M.; Bracken-Grissom, Heather D. (2015-02-01). "Phylogenetic and transcriptomic analyses reveal the evolution of bioluminescence and light detection in marine deep-sea shrimps of the family Oplophoridae (Crustacea: Decapoda)" (in en). Molecular Phylogenetics and Evolution 83: 278–292. doi:10.1016/j.ympev.2014.11.013. ISSN 1055-7903. PMID 25482362. http://www.sciencedirect.com/science/article/pii/S1055790314004151.
- ↑ Branham, Marc. "Glow-worms, railroad-worms (Insecta: Coleoptera: Phengodidae)". Featured Creatures. University of Florida. http://entnemdept.ufl.edu/creatures/misc/beetles/glow-worms.htm.
- ↑ Viviani, Vadim R.; Bechara, Etelvino J.H. (1997). "Bioluminescence and Biological Aspects of Brazilian Railroad-Worms (Coleoptera: Phengodidae)". Annals of the Entomological Society of America 90 (3): 389–398. doi:10.1093/aesa/90.3.389. https://www.researchgate.net/publication/233631656.
- ↑ Marek, Paul; Papaj, Daniel; Yeager, Justin; Molina, Sergio; Moore, Wendy (2011). "Bioluminescent aposematism in millipedes". Current Biology 21 (18): R680–R681. doi:10.1016/j.cub.2011.08.012. PMID 21959150.
- ↑ Meyer-Rochow, V. B.; Moore, S. (1988). "Biology of Latia neritoides Gray 1850 (Gastropoda, Pulmonata, Basommatophora): the Only Light-producing Freshwater Snail in the World". Internationale Revue der Gesamten Hydrobiologie und Hydrographie 73 (1): 21–42. doi:10.1002/iroh.19880730104.
- ↑ Deheyn, Dimitri D.; Wilson, Nerida G. (2010). "Bioluminescent signals spatially amplified by wavelength-specific diffusion through the shell of a marine snail". Proceedings of the Royal Society 278 (1715): 2112–2121. doi:10.1098/rspb.2010.2203. PMID 21159673.
- ↑ Bowlby, Mark R.; Widder, Edith; Case, James (1990). "Patterns of stimulated bioluminescence in two pyrosomes (Tunicata: Pyrosomatidae)". Biological Bulletin 179 (3): 340–350. doi:10.2307/1542326. PMID 29314963. https://www.biodiversitylibrary.org/part/24992.
- ↑ Encyclopedia of the Aquatic World. Marshall Cavendish. January 2004. p. 1115. ISBN 978-0-7614-7418-0. https://books.google.com/books?id=8UfQr_CqFOUC&pg=PA1115.
- ↑ Copeland, J.; Daston, M. M. (1989). "Bioluminescence in the terrestrial snail Quantula (Dyakia) striata". Malacologia 30 (1–2): 317–324. https://archive.org/stream/malacologia301989inst#page/316/mode/2up.
- ↑ Young, Richard Edward (October 1983). "Oceanic Bioluminescence: an Overview of General Functions". Bulletin of Marine Science 33 (4): 829–845. http://www.ingentaconnect.com/content/umrsmas/bullmar/1983/00000033/00000004/art00003.
- ↑ Martin, R. Aidan. "Biology of Sharks and Rays: Cookiecutter Shark". ReefQuest Centre for Shark Research. http://www.elasmo-research.org/education/ecology/deepsea-cookiecutter.htm.
- ↑ Milius, S. (1 August 1998). "Glow-in-the-dark shark has killer smudge". Science News. http://www.sciencenews.org/pages/sn_arc98/8_1_98/fob5.htm.
- ↑ Eisner, Thomas; Goetz, Michael A.; Hill, David E.; Smedley, Scott R.; Meinwald, Jarrold (1997). "Firefly "femmes fatales" acquire defensive steroids (lucibufagins) from their firefly prey". Proceedings of the National Academy of Sciences of the United States of America 94 (18): 9723–9728. doi:10.1073/pnas.94.18.9723. PMID 9275191. Bibcode: 1997PNAS...94.9723E.
- ↑ Sullivan, Rachel (16 July 2014). "Out of the darkness". ABC Science. http://www.abc.net.au/science/photos/2014/07/16/4032762.htm?xml=4032762.mediarss.xml#bigpicturepos.
- ↑ Greven, Hartmut; Zwanzig, Nadine (2013). "Courtship, Mating, and Organisation of the Pronotum in the Glowspot Cockroach Lucihormetica verrucosa (Brunner von Wattenwyl, 1865) (Blattodea: Blaberidae)". Entomologie Heute 25: 77–97. https://www.researchgate.net/publication/258845997.
- ↑ Merritt, David J. (2013). "Standards of evidence for bioluminescence in cockroaches". Naturwissenschaften 100 (7): 697–698. doi:10.1007/s00114-013-1067-9. PMID 23740173. Bibcode: 2013NW....100..697M. https://www.researchgate.net/publication/237060004.
- ↑ 71.0 71.1 71.2 Herring, Peter J.; Cope, Celia (December 2005). "Red bioluminescence in fishes: on the suborbital photophores of Malacosteus, Pachystomias and Aristostomias" (in en). Marine Biology 148 (2): 383–394. doi:10.1007/s00227-005-0085-3. ISSN 0025-3162. http://link.springer.com/10.1007/s00227-005-0085-3.
- ↑ Widder, Edith A.; Latz, Michael I.; Herring, Peter J.; Case, James F. (1984-08-03). "Far Red Bioluminescence from Two Deep-Sea Fishes" (in en). Science 225 (4661): 512–514. doi:10.1126/science.225.4661.512. ISSN 0036-8075. PMID 17750854. Bibcode: 1984Sci...225..512W. https://www.science.org/doi/10.1126/science.225.4661.512.
- ↑ 73.0 73.1 73.2 Haddock, Steven H. D.; Dunn, Casey W.; Pugh, Philip R.; Schnitzler, Christine E. (2005-07-08). "Bioluminescent and Red-Fluorescent Lures in a Deep-Sea Siphonophore" (in en). Science 309 (5732): 263. doi:10.1126/science.1110441. ISSN 0036-8075. PMID 16002609. https://www.science.org/doi/10.1126/science.1110441.
- ↑ 74.0 74.1 Bone, Q. (2008). Biology of fishes. Richard H. Moore (3rd ed.). New York: Taylor & Francis. ISBN 978-0-203-88522-2. OCLC 244632464. https://www.worldcat.org/oclc/244632464.
- ↑ Koo, J.; Kim, Y.; Kim, J.; Yeom, M.; Lee, I. C.; Nam, H. G. (2007). "A GUS/Luciferase Fusion Reporter for Plant Gene Trapping and for Assay of Promoter Activity with Luciferin-Dependent Control of the Reporter Protein Stability". Plant and Cell Physiology 48 (8): 1121–31. doi:10.1093/pcp/pcm081. PMID 17597079.
- ↑ Nordgren, I. K.; Tavassoli, A. (2014). "A bidirectional fluorescent two-hybrid system for monitoring protein-protein interactions". Molecular BioSystems 10 (3): 485–490. doi:10.1039/c3mb70438f. PMID 24382456. http://pubs.rsc.org/en/content/articlepdf/2014/mb/c3mb70438f.
- ↑ Xiong, Yan Q.; Willard, Julie; Kadurugamuwa, Jagath L.; Yu, Jun; Francis, Kevin P.; Bayer, Arnold S. (2004). "Real-Time in Vivo Bioluminescent Imaging for Evaluating the Efficacy of Antibiotics in a Rat Staphylococcus aureus Endocarditis Model". Antimicrobial Agents and Chemotherapy 49 (1): 380–7. doi:10.1128/AAC.49.1.380-387.2005. PMID 15616318.
- ↑ Di Rocco, Giuliana; Gentile, Antonietta; Antonini, Annalisa; Truffa, Silvia; Piaggio, Giulia; Capogrossi, Maurizio C.; Toietta, Gabriele (1 September 2012). "Analysis of biodistribution and engraftment into the liver of genetically modified mesenchymal stromal cells derived from adipose tissue". Cell Transplantation 21 (9): 1997–2008. doi:10.3727/096368911X637452. PMID 22469297. http://opendepot.org/2024/1/DiRocco2012.pdf.[yes|permanent dead link|dead link}}]
- ↑ Zhao, Dawen; Richer, Edmond; Antich, Peter P.; Mason, Ralph P. (2008). "Antivascular effects of combretastatin A4 phosphate in breast cancer xenograft assessed using dynamic bioluminescence imaging and confirmed by MRI". The FASEB Journal 22 (7): 2445–51. doi:10.1096/fj.07-103713. PMID 18263704. PMC 4426986. http://www.newswise.com/articles/view/541186/.
- ↑ Ow, D. W.; Wood, K. V.; DeLuca, M.; de Wet, J. R.; Helinski, D. R.; Howell, S.H. (1986). "Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants". Science (American Association for the Advancement of Science) 234 (4778): 856–859. doi:10.1126/science.234.4778.856. ISSN 0036-8075. PMID 17758108. Bibcode: 1986Sci...234..856O.
- ↑ Altura, M.A.; Heath-Heckman, E.A.; Gillette, A.; Kremer, N.; Krachler, A.M.; Brennan, C.; Ruby, E.G.; Orth, K. et al. (2013). "The first engagement of partners in the Euprymna scolopes-Vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells". Environmental Microbiology 15 (11): 2937–50. doi:10.1111/1462-2920.12179. PMID 23819708.
- ↑ "Comprehensive Squid-Vibrio Publications List". University of Wisconsin-Madison. http://labs.medmicro.wisc.edu/mcfall-ngai/allpublications.html.
- ↑ Ludwig Institute for Cancer Research (21 April 2003). "Firefly Light Helps Destroy Cancer Cells; Researchers Find That The Bioluminescence Effects of Fireflies May Kill Cancer Cells From Within". Science Daily. https://www.sciencedaily.com/releases/2003/04/030421084227.htm.
- ↑ Monteiro, Jorge H.S.K.; Sobrinho, Josiane A.; de Bettancourt-Dias, Ana (2021). "Chapter 13. Luminescence Imaging of Cancer Cells". Metal Ions in Bio-Imaging Techniques. Springer. pp. 371–401. doi:10.1515/9783110685701-019.
- ↑ Bioluminescence Questions and Answers. Siobiolum.ucsd.edu. Retrieved on 20 October 2011.
- ↑ (4 May 2013) One Per Cent: Grow your own living lights The New Scientist, Issue 2915, Retrieved 7 May 2013
- ↑ Dr. Chris Riley, "Glowing plants reveal touch sensitivity", BBC 17 May 2000.
- ↑ Halverson, Nic (15 August 2013). "Bacteria-Powered Light Bulb Is Electricity-Free". http://news.discovery.com/tech/alternative-power-sources/bacteria-powered-light-bulb-is-electricity-free-130815.htm.
- ↑ Swaminathan, Miep. "Philips launches 'Microbial Home' new forward looking design concepts". http://www.philips.com/a-w/about/news/archive/design/news/press/2011/Philips_launches%20_Microbial_Home_new_forward_looking_design_concepts.html.[yes|permanent dead link|dead link}}]
- ↑ Cha, Bonnie (28 November 2011). "Philips Bio-light creates mood lighting with bacteria". https://www.cnet.com/news/philips-bio-light-creates-mood-lighting-with-bacteria/.
- ↑ "E.glowli Cambridge: Parts submitted". iGEM. http://2010.igem.org/Team:Cambridge/BioBricks.
- ↑ 92.0 92.1 Marcellin, Frances (26 February 2016). "Glow-in-the-dark bacterial lights could illuminate shop windows 2016". New Scientist.
- ↑ "Glowee: A vision of night-time lighting". Electricite de France. 2015. http://pulse.edf.com/en/glowee-a-vision-of-night-time-lighting.
- ↑ 94.0 94.1 "Glow-in-the-dark bacterial lights could illuminate shop windows". New Scientist. 26 February 2016. https://www.newscientist.com/article/2078921-glow-in-the-dark-bacterial-lights-could-illuminate-shop-windows/.
- ↑ Mitiouchkina, Tatiana; Mishin, Alexander S.; Somermeyer, Louisa Gonzalez; Markina, Nadezhda M.; Chepurnyh, Tatiana V.; Guglya, Elena B.; Karataeva, Tatiana A.; Palkina, Kseniia A. et al. (27 April 2020). "Plants with genetically encoded autoluminescence". Nature Biotechnology 38 (8): 944–946. doi:10.1038/s41587-020-0500-9. ISSN 1546-1696. PMID 32341562.
- ↑ 96.0 96.1 Arroyo, Máira Gazzola; Ferreira, Adriano Menis; Frota, Oleci Pereira; Rigotti, Marcelo Alessandro; de Andrade, Denise; Brizzotti, Natalia Seron; Peresi, Jacqueline Tanury Macruz; Castilho, Elza Maria et al. (2017-06-30). "Effectiveness of ATP bioluminescence assay for presumptive identification of microorganisms in hospital water sources". BMC Infectious Diseases 17 (1): 458. doi:10.1186/s12879-017-2562-y. ISSN 1471-2334. PMID 28666419.
- ↑ Eed, Heba Ramadan; Abdel-Kader, Nora S.; El Tahan, Mahmoud Helmy; Dai, Tianhong; Amin, Rehab (2016). "Bioluminescence-Sensing Assay for Microbial Growth Recognition". Journal of Sensors 2016: 1–5. doi:10.1155/2016/1492467. ISSN 1687-725X.
- ↑ Tannous, Bakhos A (April 2009). "Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo". Nature Protocols 4 (4): 582–591. doi:10.1038/nprot.2009.28. ISSN 1754-2189. PMID 19373229. PMC 2692611. http://dx.doi.org/10.1038/nprot.2009.28.
Further reading
- Victor Benno Meyer-Rochow (2009) Bioluminescence in Focus – a collection of illuminating essays Research Signpost: ISBN 978-81-308-0357-9
- Shimomura, Osamu (2006). Bioluminescence: Chemical Principles and Methods. Word Scientific Publishing. ISBN 981-256-801-8.
- Lee, John (2016). "Bioluminescence, the Nature of the Light." The University of Georgia Libraries. http://hdl.handle.net/10724/20031
- Wilson, T.; Hastings, J.W. (1998). "Bioluminescence". Annual Review of Cell and Developmental Biology 14: 197–230. doi:10.1146/annurev.cellbio.14.1.197. PMID 9891783.
- Anctil, Michel (2018). Luminous Creatures: The History and Science of Light Production in Living Organisms. McGill-Queen's University Press. ISBN 978-0-7735-5312-5
External links
- BBC: Red tide: Electric blue waves wash California shore
- MBARI: Gonyaulax Bioluminescence
- UF/IFAS: glow-worms
- TED: Glowing life in an underwater world (video)
- Smithsonian Ocean Portal: Bioluminescent animals photo gallery
- National Geographic: Bioluminescence
- Annual Review of Marine Science: Bioluminescence in the Sea
- Canon Australia – Tips on How to Photograph Bioluminescence
- The New York City American Natural History Museum's "Creatures of Light: Nature's Bioluminescence" 2022 Featured Exhibit (in concert with the Ottawa, Canada-based Canadian Museum of Nature and Chicago 's Field Museum of Natural History) webpage: [2]
 |
 KSF
KSF

