Phosphonium
Topic: Physics
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
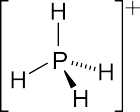
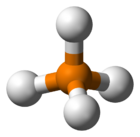
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula PR+4 (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.[1]
Types of phosphonium cations
Protonated phosphines
The parent phosphonium is PH+4 as found in the iodide salt, phosphonium iodide. Salts of the parent PH+4 are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakis(hydroxymethyl)phosphonium chloride:
- PH3 + HCl + 4 CH2O → P(CH2OH)+4Cl−
Many organophosphonium salts are produced by protonation of primary, secondary, and tertiary phosphines:
- PR3 + H+ → HPR+3
The basicity of phosphines follows the usual trends, with R = alkyl being more basic than R = aryl.[2]
Tetraorganophosphonium cations
The most common phosphonium compounds have four organic substituents attached to phosphorus. The quaternary phosphonium cations include tetraphenylphosphonium, (C6H5)4P+ and tetramethylphosphonium P(CH3)+4.

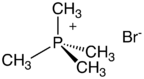
Quaternary phosphonium cations (PR+4) are produced by alkylation of organophosphines.[3] For example, the reaction of triphenylphosphine with methyl bromide gives methyltriphenylphosphonium bromide:
- PPh3 + CH3Br → [CH3PPh3]+Br−
The methyl group in such phosphonium salts is mildly acidic, with a pKa estimated to be near 15:[5]
- [CH3PPh3]+ + base → CH2=PPh3 + [Hbase]+
This deprotonation reaction gives Wittig reagents.[6]
Solid phosphorus pentachloride is an ionic compound, formulated PCl+4PCl−6, that is, a salt containing the tetrachlorophosphonium cation.[7][8] Dilute solutions dissociate according to the following equilibrium:
- PCl5 ⇌ PCl+4 + Cl−
Triphenylphosphine dichloride (Ph3PCl2) exists both as the pentacoordinate phosphorane and as the chlorotriphenylphosphonium chloride, depending on the medium.[9] The situation is similar to that of PCl5. It is an ionic compound (PPh3Cl)+Cl− in polar solutions and a molecular species with trigonal bipyramidal molecular geometry in apolar solution.[10]
Alkoxyphosphonium salts: Arbuzov reaction
The Michaelis–Arbuzov reaction is the chemical reaction of a trivalent phosphorus ester with an alkyl halide to form a pentavalent phosphorus species and another alkyl halide. Commonly, the phosphorus substrate is a phosphite ester (P(OR)3) and the alkylating agent is an alkyl iodide.[11] center|600px|The mechanism of the Michaelis–Arbuzov reaction
Uses
Textile finishes
Tetrakis(hydroxymethyl)phosphonium chloride has industrial importance in the production of crease-resistant and flame-retardant finishes on cotton textiles and other cellulosic fabrics.[12][13] A flame-retardant finish can be prepared from THPC by the Proban Process,[14] in which THPC is treated with urea. The urea condenses with the hydroxymethyl groups on THPC. The phosphonium structure is converted to phosphine oxide as the result of this reaction.[15]
Phase-transfer catalysts and precipitating agents
Organic phosphonium cations are lipophilic and can be useful in phase transfer catalysis, much like quaternary ammonium salts. Salts or inorganic anions and tetraphenylphosphonium (PPh+4) are soluble in polar organic solvents. One example is the perrhenate (PPh4[ReO4]).[16]
Reagents for organic synthesis
Wittig reagents are used in organic synthesis. They are derived from phosphonium salts. A strong base such as butyllithium or sodium amide is required for the deprotonation:
- [Ph3P+CH2R]X− + C4H9Li → Ph3P=CHR + LiX + C4H10
One of the simplest ylides is methylenetriphenylphosphorane (Ph3P=CH2).[6]
The compounds Ph3PX2 (X = Cl, Br) are used in the Kirsanov reaction.[17] The Kinnear–Perren reaction is used to prepare alkylphosphonyl dichlorides (RP(O)Cl2) and esters (RP(O)(OR′)2). A key intermediate are alkyltrichlorophosphonium salts, obtained by the alkylation of phosphorus trichloride:[18]
- RCl + PCl3 + AlCl3 → [RPCl3]+AlCl−4
Ammonia production for "green hydrogen"
The main industrial procedure for the production of ammonia today is the thermal Haber-Bosch process, which generally uses fossil gas as a source of hydrogen, which is then combined with nitrogen to produce ammonia. In 2021, Professor Doug MacFarlane and collaborators Alexandr Simonov and Bryan Suryanto of Monash University devised a method of producing green ammonia that has the potential to make Haber-Bosch plants obsolete.[19] Their process is similar to the electrolysis approach for producing hydrogen. While working with local company Verdant, which wanted to make bleach from saltwater by electrolysis, Suryanto discovered that a tetraalkyl phosphonium salt allowed the efficient production of ammonia at room temperature.[20]
See also
- Ammonium (NH+4)
- Arsonium (AsH+4)
- Hydronium (H3O+)
- Onium compounds
- Organophosphorus chemistry
References
- ↑ Corbridge, D. E. C. (1995). Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier. ISBN 978-0-444-89307-9.
- ↑ Li, T.; Lough, A. J.; Morris, R. H. (2007). "An Acidity Scale of Tetrafluoroborate Salts of Phosphonium and Iron Hydride Compounds in [D2]Dichloromethane". Chem. Eur. J. 13 (13): 3796–3803. doi:10.1002/chem.200601484. PMID 17245785.
- ↑ 3.0 3.1 H.-F. Klein (1978). "Trimethylphosphonium Methylide (Trimethyl Methylenephosphorane)". Inorganic Syntheses. Inorganic Syntheses. 18. pp. 138–140. doi:10.1002/9780470132494.ch23. ISBN 9780470132494.
- ↑ Finch, A.; Fitch, A.N.; Gates, P.N. (1993). "Crystal and Molecular structure of a metastable modification of phosphorus pentachloride". Journal of the Chemical Society, Chemical Communications (11): 957–958. doi:10.1039/c39930000957.
- ↑ Ling-Chung, Sim; Sales, Keith D.; Utley, James H. P. (1990). "Measurement of pKa Values for Phosphonium Salts via the Kinetics of Proton Transfer to an Electrogenerated Base". Journal of the Chemical Society, Chemical Communications (9): 662. doi:10.1039/C39900000662.
- ↑ 6.0 6.1 Wittig; Schoellkopf, U. (1960). "Methylenecyclohexane". Organic Syntheses 40: 66. doi:10.15227/orgsyn.040.0066.. Describes Ph3P=CH2.
- ↑ Holleman, A. F.; Wiber, E.; Wiberg, N. (2001). Inorganic Chemistry. Academic Press. ISBN 978-0-12-352651-9.
- ↑ Suter, R. W.; Knachel, H. C.; Petro, V. P.; Howatson, J. H.; Shore, S. G. (1978). "Nature of Phosphorus(V) Chloride in Ionizing and Nonionizing Solvents". Journal of the American Chemical Society 95 (5): 1474–1479. doi:10.1021/ja00786a021.
- ↑ S. M. Godfrey; C. A. McAuliffe; R. G. Pritchard; J. M. Sheffield (1996). "An X-ray crystallorgraphic study of the reagent Ph3PCl2; not charge-transfer, R3P–Cl–Cl, trigonal bipyramidal or [R3PCl]Cl but an unusual dinuclear ionic species, [Ph3PCl+⋯Cl–⋯+CIPPh3]Cl containing long Cl–Cl contacts". Chemical Communications (22): 2521–2522. doi:10.1039/CC9960002521.
- ↑ Jennings, EV; Nikitin, K; Ortin, Y; Gilheany, DG (2014). "Degenerate Nucleophilic Substitution in Phosphonium Salts". J. Am. Chem. Soc. 136 (46): 16217–16226. doi:10.1021/ja507433g. PMID 25384344.
- ↑ Bhattacharya, A. K.; Thyagarajan, G. (1981). "Michaelis–Arbuzov rearrangement". Chem. Rev. 81 (4): 415–430. doi:10.1021/cr00044a004.
- ↑ Weil, Edward D.; Levchik, Sergei V. (2008). "Flame Retardants in Commercial Use or Development for Textiles". J. Fire Sci. 26 (3): 243–281. doi:10.1177/0734904108089485.
- ↑ Svara, Jürgen; Weferling, Norbert ; Hofmann, Thomas. Phosphorus Compounds, Organic. Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons, Inc., 2008 doi:10.1002/14356007.a19_545.pub2
- ↑ "Frequently asked questions: What is the PROBAN® process?". Rhodia Proban. http://www.rhodia-proban.com/uk/faq.asp.
- ↑ Reeves, Wilson A.; Guthrie, John D. (1956). "Intermediate for Flame-Resistant Polymers-Reactions of Tetrakis(hydroxymethyl)phosphonium Chloride". Industrial and Engineering Chemistry 48 (1): 64–67. doi:10.1021/ie50553a021.
- ↑ Dilworth, J. R.; Hussain, W.; Hutson, A. J.; Jones, C. J.; McQuillan, F. S. (1996). "Tetrahalo Oxorhenate Anions". Inorganic Syntheses. Inorganic Syntheses. XXXI. pp. 257–262. doi:10.1002/9780470132623.ch42. ISBN 9780470132623.
- ↑ Studies in Organophosphorus Chemistry. I. Conversion of Alcohols and Phenols to Halides by Tertiary Phosphine Dihalides G. A. Wiley, R. L. Hershkowitz, B. M. Rein, B. C. Chung J. Am. Chem. Soc., 1964, 86 (5), pp 964–965 doi:10.1021/ja01059a073
- ↑ Svara, J.; Weferling, N.; Hofmann, T.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_545.pub2.
- ↑ Breakthrough brings green ammonia production closer to reality
- ↑ Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle
 |
 KSF
KSF