Photo-oxidation of polymers
Topic: Physics
 From HandWiki - Reading time: 19 min
From HandWiki - Reading time: 19 min


In polymer chemistry photo-oxidation (sometimes: oxidative photodegradation) is the degradation of a polymer surface due to the combined action of light and oxygen.[1] It is the most significant factor in the weathering of plastics.[2] Photo-oxidation causes the polymer chains to break (chain scission), resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.
Technologies have been developed to both accelerate and inhibit this process. For example, plastic building components like doors, window frames and gutters are expected to last for decades, requiring the use of advanced UV-polymer stabilizers. Conversely, single-use plastics can be treated with biodegradable additives to accelerate their fragmentation. Many pigments and dyes can similarly have effects due to their ability to absorb UV-energy.
Susceptible polymers
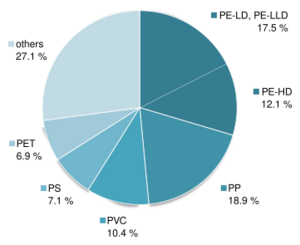
PP: polypropylene, PE: polyethylene, PVC: Polyvinyl chloride, PS: Polystyrene, PET: Polyethylene terephthalate
Susceptibility to photo-oxidation varies depending on the chemical structure of the polymer. Some materials have excellent stability, such as fluoropolymers, polyimides, silicones and certain acrylate polymers. However, global polymer production is dominated by a range of commodity plastics which account for the majority of plastic waste. Of these polyethylene terephthalate (PET) has only moderate UV resistance and the others, which include polystyrene, polyvinyl chloride (PVC) and polyolefins like polypropylene (PP) and polyethylene (PE) are all highly susceptible.
Photo-oxidation is a form of photodegradation and begins with formation of free radicals on the polymer chain, which then react with oxygen in chain reactions. For many polymers the general autoxidation mechanism is a reasonable approximation of the underlying chemistry. The process is autocatalytic, generating increasing numbers of radicals and reactive oxygen species. These reactions result in changes to the molecular weight (and molecular weight distribution) of the polymer and as a consequence the material becomes more brittle. The process can be divided into four stages:
- Initiation the process of generating the initial free radical.
- Propagation the conversion of one active species to another
- Chain branching steps which end with more than one active species being produced. The photolysis of hydroperoxides is the main example.
- Termination steps in which active species are removed, for instance by radical disproportionation
Photo-oxidation can occur simultaneously with other processes like thermal degradation, and each of these can accelerate the other.
Polyolefins
Polyolefins such as polyethylene and polypropylene are susceptible to photo-oxidation and around 70% of light stabilizers produced world-wide are used in their protection, despite them representing only around 50% of global plastic production.[1] Aliphatic hydrocarbons can only adsorb high energy UV-rays with a wavelength below ~250 nm, however the Earth’s atmosphere and ozone layer screen out such rays, with the normal minimum wavelength being 280–290 nm.[3] The bulk of the polymer is therefore photo-inert and degradation is instead attributed to the presence of various impurities, which are introduced during the manufacturing or processing stages. These include hydroperoxide and carbonyl groups, as well as metal salts such as catalyst residues.
All of these species act as photoinitiators.[4] The organic hydroperoxide and carbonyl groups are able to absorb UV light above 290 nm whereupon they undergo photolysis to generate radicals.[5] Metal impurities act as photocatalysts,[6] although such reactions can be complex.[7][8] It has also been suggested that polymer-O2 charge-transfer complexes are involved.[9][10] Initiation generates radical-carbons on the polymer chain, sometimes called macroradicals (P•).
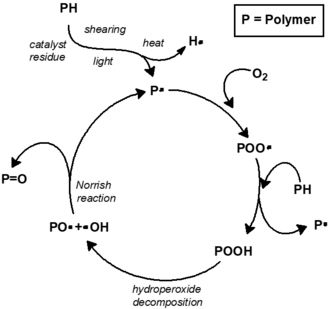
Chain initiation
Chain propagation
Chain branching
Termination
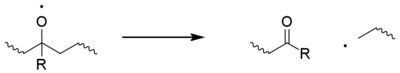
Classically the carbon-centred macroradicals (P•) rapidly react with oxygen to form hydroperoxyl radicals (POO•), which in turn abstract an H atom from the polymer chain to give a hydroperoxide (POOH) and a fresh macroradical. Hydroperoxides readily undergo photolysis to give an alkoxyl macroradical radical (PO•) and a hydroxyl radical (HO•), both of which may go on to form new polymer radicals via hydrogen abstraction. Non-classical alternatives to these steps have been proposed.[11] The alkoxyl radical may also undergo beta scission,[12] generating a acyl-ketone and macroradical. This is considered to be the main cause of chain breaking in polypropylene.[13]
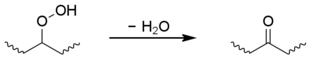
Secondary hydroperoxides can also undergo an intramolecular reaction to give a ketone group, although this is limited to polyethylene.[1][14][15][16]
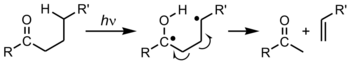
The ketones generated by these processes are themselves photo-active, although much more weakly. At ambient temperatures they undergo Type II Norrish reactions with chain scission.[17] They may also absorb UV-energy, which they can then transfer to O2, causing it to enter its highly reactive singlet state.[18] Singlet oxygen is a potent oxidising agent can go on to form cause further degradation.
Polystyrene

For polystyrene the complete mechanism of photo-oxidation is still a matter of debate, as different pathways may operate concurrently[20] and vary according to the wavelength of the incident light.[21][22] Regardless, there is agreement on the major steps.[19]
Pure polystyrene should not be able to absorb light with a wavelength below ~280 nm and initiation is explained though photo-labile impurities (hydroperoxides) and charge transfer complexes,[23] all of which are able to absorb normal sunlight.[24] Charge-transfer complexes of oxygen and polystyrene phenyl groups absorb light to form singlet oxygen, which acts as a radical initiator. [23] Carbonyl impurities in the polymer (c.f. acetophenone) also absorb light in the near ultraviolet range (300 to 400 nm), forming excited ketones able to abstract hydrogen atoms directly from the polymer.[24] Hyroperoxide undergoes photolysis to form hydroxyl and alkoxyl radicals.
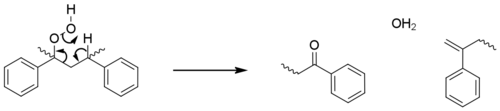
These initiation steps generate macroradicals at tertiary sites, as these are more stabilised. The propagation steps are essentially identical to those seen for polyolefins; with oxidation, hydrogen abstraction and photolysis leading to beta scission reactions and increasing numbers of radicals. These steps account for the majority of chain-breaking, however in a minor pathway the hydroperoxide reacts directly with polymer to form a ketone group (acetophenone) and a terminal alkene without the formation of additional radicals.[25]
Polystyrene is observed to yellow during photo-oxidation, which is attributed to the formation of polyenes from these terminal alkenes.[25]
Polyvinyl chloride (PVC)
Pure organochlorides like polyvinyl chloride (PVC) do not absorb any light above 220 nm. The initiation of photo-oxidation is instead caused by various irregularities in the polymer chain, such as structural defects[26][27] as well as hydroperoxides, carbonyl groups, and double bonds.[28] Hydroperoxides formed during processing are the most important initiator to begin with,[29] however their concentration decreases during photo-oxidation whereas carbonyl concentration increases,[30] as such carbonyls may become the primary initiator over time.[29][31][32]
Propagation steps involve the hydroperoxyl radical, which can abstract hydrogen from both hydrocarbon (-CH2-) and organochloride (-CH2Cl-) sites in the polymer at comparable rates.[29][31] Radicals formed at hydrocarbon sites rapidly convert to alkenes with loss of radical chlorine. This forms allylic hydrogens (shown in red) which are more susceptible to hydrogen abstraction leading to the formation of polyenes in zipper-like reactions.
When the polyenes contain at least eight conjugated double bonds they become coloured, leading to yellowing and eventual browning of the material. This is off-set slightly by longer polyenes being photobleached with atmospheric oxygen,[33] however PVC does eventually discolour unless polymer stabilisers are present. Reactions at organochloride sites proceed via the usual hydroperoxyl and hydroperoxide before photolysis yields the α-chloro-alkoxyl radical. This species can undergo various reactions to give carbonyls, peroxide cross-links and beta scission products.[34]

Poly(ethylene terephthalate) - (PET)
Unlike most other commodity plastics polyethylene terephthalate (PET) is able to absorb the near ultraviolet rays in sunlight. Absorption begins at 360 nm, becoming stronger below 320 nm and is very significant below 300 nm.[1][35][36] Despite this PET has better resistance to photo-oxidation than other commodity plastics, this is due to a poor quantum yield or the absorption.[37] The degradation chemistry is complicated due to simultaneous photodissociation (i.e. not involving oxygen) and photo-oxidation reactions of both the aromatic and aliphatic parts of the molecule. Chain scission is the dominant process, with chain branching and the formation of coloured impurities being less common. Carbon monoxide, carbon dioxide, and carboxylic acids are the main products.[35][36] The photo-oxidation of other linear polyesters such as polybutylene terephthalate and polyethylene naphthalate proceeds similarly.
Photodissociation involves the formation of an excited terephthalic acid unit which undergoes Norrish reactions. The type I reaction dominates, which cause chain scission at the carbonyl unit to give a range of products.[1][38]
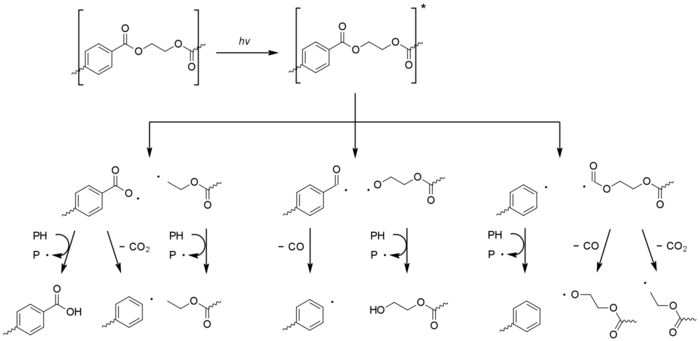
Type II Norrish reactions are less common but give rise to acetaldehyde by way of vinyl alcohol esters.[36] This has an exceedingly low odour and taste threshold and can cause an off-taste in bottled water.[39]
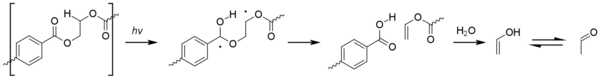
Radicals formed by photolysis may initiate the photo-oxidation in PET. Photo-oxidation of the aromatic terephthalic acid core results in its step-wise oxidation to 2,5-dihydroxyterephthalic acid. The photo-oxidation process at aliphatic sites is similar to that seen for polyolefins, with the formation of hydroperoxide species eventually leading to beta-scission of the polymer chain.[1]
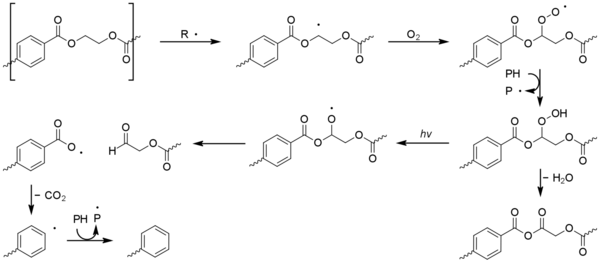
Secondary factors
Environment
Perhaps surprisingly, the effect of temperature is often greater than the effect of UV exposure.[5] This can be seen in terms of the Arrhenius equation, which shows that reaction rates have an exponential dependence on temperature. By comparison the dependence of degradation rate on UV exposure and the availability of oxygen is broadly linear. As the oceans are cooler than land plastic pollution in the marine environment degrades more slowly.[40][41] Materials buried in landfill do not degrade by photo-oxidation at all, though they may gradually decay by other processes.
Mechanical stress can effect the rate of photo-oxidation[42] and may also accelerate the physical breakup of plastic objects. Stress can be caused by mechanical load (tensile and shear stresses) or even by temperature cycling, particularly in composite systems consisting of materials with differing temperature coefficients of expansion. Similarly, sudden rainfall can cause thermal stress.
Effects of dyes and other additives
Dyes and pigments are used in polymer materials to provide colour, however they can also effect the rate of photo-oxidation. Many absorb UV rays and in so doing protect the polymer, however absorption can cause the dyes to enter an excited state where they may attack the polymer or transfer energy to O2 to form damaging singlet oxygen. Cu-phthalocyanine is an example, it strongly absorbs UV light however the excited Cu-phthalocyanine may act as a photoinitiator by abstracting hydrogen atoms from the polymer.[43] Its interactions may become even more complicated when other additives are present.[44] Fillers such as carbon black can screen out UV light, effectively stabilisers the polymer, whereas flame retardants tend to cause increased levels of photo-oxidation.[45]
Additives to enhance degradation
Biodegradable additives may be added to polymers to accelerate their degradation. In the case of photo-oxidation OXO-biodegradation additives are used.[46] These are transition metal salts such as iron (Fe), manganese (Mn), and cobalt (Co). Fe complexes increase the rate of photooxidation by promoting the homolysis of hydroperoxides via Fenton reactions.
The use of such additives has been controversial due to concerns that treated plastics do not fully biodegrade and instead result in the accelerated formation of microplastics.[47] Oxo-plastics would be difficult to distinguish from untreated plastic but their inclusion during plastic recycling can create a destabilised product with fewer potential uses,[48][49] potentially jeopardising the business case for recycling any plastic. OXO-biodegradation additives were banned in the EU in 2019[50]
Prevention
UV attack by sunlight can be ameliorated or prevented by adding anti-UV polymer stabilizers, usually prior to shaping the product by injection moulding. UV stabilizers in plastics usually act by absorbing the UV radiation preferentially, and dissipating the energy as low-level heat. The chemicals used are similar to those in sunscreen products, which protect skin from UV attack. They are used frequently in plastics, including cosmetics and films. Different UV stabilizers are utilized depending upon the substrate, intended functional life, and sensitivity to UV degradation. UV stabilizers, such as benzophenones, work by absorbing the UV radiation and preventing the formation of free radicals. Depending upon substitution, the UV absorption spectrum is changed to match the application. Concentrations normally range from 0.05% to 2%, with some applications up to 5%.
Frequently, glass can be a better alternative to polymers when it comes to UV degradation. Most of the commonly used glass types are highly resistant to UV radiation. Explosion protection lamps for oil rigs for example can be made either from polymer or glass. Here, the UV radiation and rough weathers belabor the polymer so much, that the material has to be replaced frequently.
Poly(ethylene-naphthalate) (PEN) can be protected by applying a zinc oxide coating, which acts as protective film reducing the diffusion of oxygen.[51] Zinc oxide can also be used on polycarbonate (PC) to decrease the oxidation and photo-yellowing rate caused by solar radiation.[52]
Analysis
Weather testing of polymers

The photo-oxidation of polymers can be investigated by either natural or accelerated weather testing.[53] Such testing is important in determining the expected service-life of plastic items as well as the fate of waste plastic.
In natural weather testing, polymer samples are directly exposed to open weather for a continuous period of time,[54] while accelerated weather testing uses a specialized test chamber which simulates weathering by sending a controlled amount of UV light and water at a sample. A test chamber may be advantageous in that the exact weathering conditions can be controlled, and the UV or moisture conditions can be made more intense than in natural weathering. Thus, degradation is accelerated and the test is less time-consuming.
Through weather testing, the impact of photooxidative processes on the mechanical properties and lifetimes of polymer samples can be determined. For example, the tensile behavior can be elucidated through measuring the stress–strain curve for a specimen. This stress–strain curve is created by applying a tensile stress (which is measured as the force per area applied to a sample face) and measuring the corresponding strain (the fractional change in length). Stress is usually applied until the material fractures, and from this stress–strain curve, mechanical properties such as the Young’s modulus can be determined. Overall, weathering weakens the sample, and as it becomes more brittle, it fractures more easily. This is observed as a decrease in the yield strain, fracture strain, and toughness, as well as an increase in the Young’s modulus and break stress (the stress at which the material fractures).[55]
Aside from measuring the impact of degradation on mechanical properties, the degradation rate of plastic samples can also be quantified by measuring the change in mass of a sample over time, as microplastic fragments can break off from the bulk material as degradation progresses and the material becomes more brittle through chain-scission. Thus, the percentage change in mass is often measured in experiments to quantify degradation.[56]
Mathematical models can also be created to predict the change in mass of a polymer sample over the weathering process. Because mass loss occurs at the surface of the polymer sample, the degradation rate is dependent on surface area. Thus, a model for the dependence of degradation on surface area can be made by assuming that the rate of change in mass resulting from degradation is directly proportional to the surface area SA of the specimen:[57]
Here, is the density and kd is known as the specific surface degradation rate (SSDR), which changes depending on the polymer sample’s chemical composition and weathering environment. Furthermore, for a microplastic sample, SA is often approximated as the surface area of a cylinder or sphere. Such an equation can be solved to determine the mass of a polymer sample as a function of time.
Detection
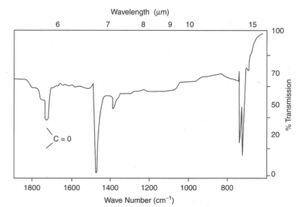
Degradation can be detected before serious cracks are seen in a product by using infrared spectroscopy,[58] which is able to detect chemical species formed by photo-oxidation. In particular, peroxy-species and carbonyl groups have distinct absorption bands.
In the example shown at left, carbonyl groups were easily detected by IR spectroscopy from a cast thin film. The product was a road cone made by rotational moulding in LDPE, which had cracked prematurely in service. Many similar cones also failed because an anti-UV additive had not been used during processing. Other plastic products which failed included polypropylene mancabs used at roadworks which cracked after service of only a few months.

The effects of degradation can also be characterized through scanning electron microscopy (SEM). For example, through SEM, defects like cracks and pits can be directly visualized, as shown at right. These samples were exposed to 840 hours of exposure to UV light and moisture using a test chamber.[56] Crack formation is often associated with degradation, such that materials that do not display significant cracking behavior, such as HDPE in the right example, are more likely to be stable against photooxidation compared to other materials like LDPE and PP. However, some plastics that have undergone photooxidation may also appear smoother in an SEM image, with some defects like grooves having disappeared afterwards. This is seen in polystyrene in the right example.
See also
- Forensic polymer engineering
- Photodegradation
- Polymer degradation
- Stress corrosion cracking
- Thermal degradation of polymers
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Zweifel, Hans; Maier, Ralph D.; Schiller, Michael (2009). Plastics additives handbook (6th ed.). Munich: Hanser. ISBN 978-3-446-40801-2.
- ↑ Feldman, D. (1 October 2002). "Polymer Weathering: Photo-Oxidation". Journal of Polymers and the Environment 10 (4): 163–173. doi:10.1023/A:1021148205366.
- ↑ Solar spectral irradiance (1st ed.). Vienna: Commission internationale de l'eclairage. 1989. ISBN 9783900734220.
- ↑ Amin, M.U.; Scott, G.; Tillekeratne, L.M.K. (January 1975). "Mechanism of the photo-initiation process in polyethylene". European Polymer Journal 11 (1): 85–89. doi:10.1016/0014-3057(75)90179-2.
- ↑ 5.0 5.1 Grause, Guido; Chien, Mei-Fang; Inoue, Chihiro (November 2020). "Changes during the weathering of polyolefins". Polymer Degradation and Stability 181: 109364. doi:10.1016/j.polymdegradstab.2020.109364.
- ↑ Allen, Norman S.; Chirinos-Padron, Alfonso; Henman, Terence J. (March 1985). "Photoinitiated oxidation of polypropylene: a review". Progress in Organic Coatings 13 (2): 97–122. doi:10.1016/0033-0655(85)80020-0.
- ↑ Osawa, Zenjiro (January 1988). "Role of metals and metal-deactivators in polymer degradation". Polymer Degradation and Stability 20 (3–4): 203–236. doi:10.1016/0141-3910(88)90070-5.
- ↑ Hussain, Ikram; Atiqullah, Muhammad; Fazal, Atif; Alam, Khurshid; Hossaen, Anwar (December 2010). "Effect of selected residual Ziegler–Natta and metallocene catalysts on the UV-induced degradation of unstabilized ethylene homopolymer films". Polymer Degradation and Stability 95 (12): 2289–2299. doi:10.1016/j.polymdegradstab.2010.09.004.
- ↑ Gijsman, Pieter; Sampers, Jacq (January 1997). "The influence of oxygen pressure and temperature on the UV-degradation chemistry of polyethylene". Polymer Degradation and Stability 58 (1–2): 55–59. doi:10.1016/S0141-3910(97)00012-8.
- ↑ Chien, J. C. W. (December 1965). "On the Possible Initiation of Photooxidation by Charge-Transfer Excitation". The Journal of Physical Chemistry 69 (12): 4317–4325. doi:10.1021/j100782a040.
- ↑ Smith, Leesa M.; Aitken, Heather M.; Coote, Michelle L. (18 September 2018). "The Fate of the Peroxyl Radical in Autoxidation: How Does Polymer Degradation Really Occur?". Accounts of Chemical Research 51 (9): 2006–2013. doi:10.1021/acs.accounts.8b00250. PMID 30016062.
- ↑ Gray, Peter; Williams, Alan (1 April 1959). "The Thermochemistry And Reactivity Of Alkoxyl Radicals". Chemical Reviews 59 (2): 239–328. doi:10.1021/cr50026a002.
- ↑ Carlsson, D. J.; Wiles, D. M. (November 1969). "The Photodegradation of Polypropylene Films. III. Photolysis of Polypropylene Hydroperoxides". Macromolecules 2 (6): 597–606. doi:10.1021/ma60012a007. Bibcode: 1969MaMol...2..597C.
- ↑ Costa, L.; Luda, M.P.; Trossarelli, L. (January 1997). "Ultra high molecular weight polyethylene—II. Thermal- and photo-oxidation". Polymer Degradation and Stability 58 (1–2): 41–54. doi:10.1016/S0141-3910(97)00010-4. https://d1wqtxts1xzle7.cloudfront.net/48805104/s0141-3910_2897_2900010-420160913-5432-1vr1njl.pdf?1473786853=&response-content-disposition=inline%3B+filename%3DUltra_high_molecular_weight_polyethylene.pdf&Expires=1611669970&Signature=ShBg2mTuVAOetVeFQAj9lC0ObsZwZLf-ph-kWLGAZNR2VOxYQqdUTBpdQDYNxOXdEe9~Ay5Dnmhwdvmq9-RQ8mft54m2zUD5fWdbOqqsXLTBm3WoD37U5FHabmeX5Wplvy0agRvzoIaeOCglui7MPR5dpNKuEUBLKLck2GUDFY1SuX76W8R~kwtEeWDd-cEsEOHyKD-ECE5wtl9bwVHZGSNqb4RzFbYQGalxd78-TsUjnbqMA4tbSWAGSC6Ss0Z-zkd5UVd1k~Y2uaYVj7F1eVtTHtuTveOeAPjGw~oNOBWYPAC82dzBwX1mzZrdvfMKV7~ifYy9ooCQgAIZSIBoaQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA.[|permanent dead link|dead link}}]
- ↑ Gugumus, F. (January 1990). "Contribution to the photolysis of hydroperoxides in polyethylene". Polymer Degradation and Stability 27 (1): 19–34. doi:10.1016/0141-3910(90)90094-N.
- ↑ Gugumus, F. (March 1988). "Contribution to polyethylene photooxidation". Angewandte Makromolekulare Chemie 158 (1): 151–176. doi:10.1002/apmc.1988.051580108.
- ↑ Hartley, G. H.; Guillet, J. E. (March 1968). "Photochemistry of Ketone Polymers. I. Studies of Ethylene-Carbon Monoxide Copolymers". Macromolecules 1 (2): 165–170. doi:10.1021/ma60002a012. Bibcode: 1968MaMol...1..165H.
- ↑ Rabek, J. F.; ??anby, B. (January 1975). "Role of singlet oxygen in photo-oxidative degradation and photostabilization of polymers". Polymer Engineering and Science 15 (1): 40–43. doi:10.1002/pen.760150107.
- ↑ 19.0 19.1 Yousif, Emad; Haddad, Raghad (December 2013). "Photodegradation and photostabilization of polymers, especially polystyrene: review". SpringerPlus 2 (1): 398. doi:10.1186/2193-1801-2-398. PMID 25674392.
- ↑ Kuzina, Svetlana I.; Mikhailov, Alfa I. (November 2001). "Photo-oxidation of polymers 4. The dual mechanism of polystyrene photo-oxidation: a hydroperoxide and a photochain one". European Polymer Journal 37 (11): 2319–2325. doi:10.1016/S0014-3057(01)00028-3.
- ↑ Kuzina, S.I.; Mikhailov, A.I. (December 1993). "The photo-oxidation of polymers—1. Initiation of polystyrene photo-oxidation". European Polymer Journal 29 (12): 1589–1594. doi:10.1016/0014-3057(93)90250-J.
- ↑ Gardette, Jean-Luc; Mailhot, Bénédicte; Lemaire, Jacques (January 1995). "Photooxidation mechanisms of styrenic polymers". Polymer Degradation and Stability 48 (3): 457–470. doi:10.1016/0141-3910(95)00113-Z.
- ↑ 23.0 23.1 Rabek, Jan F.; Rånby, Bengt (February 1974). "Studies on the photooxidation mechanism of polymers. I. Photolysis and photooxidation of polystyrene". Journal of Polymer Science: Polymer Chemistry Edition 12 (2): 273–294. doi:10.1002/pol.1974.170120203. Bibcode: 1974JPoSA..12..273R.
- ↑ 24.0 24.1 Ranby, B.; Lucki, Julia (1 January 1980). "New aspects of photodegradation and photooxidation of polystyrene". Pure and Applied Chemistry 52 (2): 295–303. doi:10.1351/pac198052020295.
- ↑ 25.0 25.1 Geuskens, G.; Baeyens-Volant, D.; Delaunois, G.; Lu-Vinh, Q.; Piret, W.; David, C. (1 January 1978). "Photo-oxidation of polymers—I: A quantitative study of the chemical reactions resulting from irradiation of polystyrene at 253.7 nm in the presence of oxygen". European Polymer Journal 14 (4): 291–297. doi:10.1016/0014-3057(78)90051-4.
- ↑ Starnes, William H. (3 May 2005). "Structural defects in poly(vinyl chloride)". Journal of Polymer Science Part A: Polymer Chemistry 43 (12): 2451–2467. doi:10.1002/pola.20811. Bibcode: 2005JPoSA..43.2451S.
- ↑ Adeniyi, Jacob B.; Scott, Gerald (1 January 1987). "The effects of structural defects on the stability of poly(vinyl chloride): A critical review". Polymer Degradation and Stability 17 (2): 117–129. doi:10.1016/0141-3910(87)90099-1.
- ↑ Starnes, W. H. (8 April 1981). "Photodegradation of Polyvinyl Chloride: A Survey of Recent Studies". Photodegradation and Photostabilization of Coatings 151: 197–215. doi:10.1021/bk-1981-0151.ch014.
- ↑ 29.0 29.1 29.2 Boyd Cooray, B.; Scott, G. (February 1981). "The effect of thermal processing on PVC—Part VIII: The role of thermally formed peroxides on photo-degradation". Polymer Degradation and Stability 3 (2): 127–135. doi:10.1016/0141-3910(81)90005-7.
- ↑ Jian, Li; Dafei, Zhou; Deren, Zhao (January 1991). "The photo-degradation of PVC: Part II—Structural changes in PVC chains". Polymer Degradation and Stability 31 (1): 1–7. doi:10.1016/0141-3910(91)90091-5.
- ↑ 31.0 31.1 Decker, C. (January 1984). "Degradation of poly(vinyl chloride) by u.v. radiation—II". European Polymer Journal 20 (2): 149–155. doi:10.1016/0014-3057(84)90201-5.
- ↑ Decker, Christian; Balandier, Michel (July 1981). "Photo-oxidation of poly(vinyl chloride)". Polymer Photochemistry 1 (3): 221–232. doi:10.1016/0144-2880(81)90021-X.
- ↑ Rabek, Jan F.; Rånby, Bengt; Östensson, Bengt; Flodin, Per (15 December 1979). "Oxidation of polyene structures in poly(vinyl chloride) by molecular oxygen and singlet oxygen". Journal of Applied Polymer Science 24 (12): 2407–2413. doi:10.1002/app.1979.070241209.
- ↑ Jian, Li; Dafei, Zhou; Deren, Zhao (January 1990). "The photo-degradation of PVC: Part I—Photo-degradation in air and nitrogen". Polymer Degradation and Stability 30 (3): 335–343. doi:10.1016/0141-3910(90)90087-N.
- ↑ 35.0 35.1 Day, M.; Wiles, D. M. (January 1972). "Photochemical degradation of poly(ethylene terephthalate). II. Effect of wavelength and environment on the decomposition process". Journal of Applied Polymer Science 16 (1): 191–202. doi:10.1002/app.1972.070160117.
- ↑ 36.0 36.1 36.2 Day, M.; Wiles, D. M. (January 1972). "Photochemical degradation of poly(ethylene terephthalate). III. Determination of decomposition products and reaction mechanism". Journal of Applied Polymer Science 16 (1): 203–215. doi:10.1002/app.1972.070160118.
- ↑ Osborn, K. R. (August 1959). "The photolysis of polyethylene terephthalate". Journal of Polymer Science 38 (134): 357–367. doi:10.1002/pol.1959.1203813407. Bibcode: 1959JPoSc..38..357O.
- ↑ Sang, Tian; Wallis, Christopher J.; Hill, Gavin; Britovsek, George J.P. (August 2020). "Polyethylene terephthalate degradation under natural and accelerated weathering conditions". European Polymer Journal 136: 109873. doi:10.1016/j.eurpolymj.2020.109873.
- ↑ Nawrocki, J; Dąbrowska, A; Borcz, A (November 2002). "Investigation of carbonyl compounds in bottled waters from Poland". Water Research 36 (19): 4893–4901. doi:10.1016/S0043-1354(02)00201-4. PMID 12448533.
- ↑ Pegram, Jan E.; Andrady, Anthony L. (January 1989). "Outdoor weathering of selected polymeric materials under marine exposure conditions". Polymer Degradation and Stability 26 (4): 333–345. doi:10.1016/0141-3910(89)90112-2.
- ↑ Chamas, Ali; Moon, Hyunjin; Zheng, Jiajia; Qiu, Yang; Tabassum, Tarnuma; Jang, Jun Hee; Abu-Omar, Mahdi; Scott, Susannah L. et al. (9 March 2020). "Degradation Rates of Plastics in the Environment". ACS Sustainable Chemistry & Engineering 8 (9): 3494–3511. doi:10.1021/acssuschemeng.9b06635.
- ↑ Tyler, David R. (30 December 2004). "Mechanistic Aspects of the Effects of Stress on the Rates of Photochemical Degradation Reactions in Polymers". Journal of Macromolecular Science, Part C: Polymer Reviews 44 (4): 351–388. doi:10.1081/MC-200033682.
- ↑ "THE PHOTO-OXIDATION OF POLYMERS - A comparison with low molecular weight compounds". Pergamon Press Ltd. 1979 - Pure & Appi. Chem., Vol. 51, pp.233—240. http://www.iupac.org/publications/pac/pdf/1979/pdf/5102x0233.pdf.
- ↑ Allen, N.S.; Vasiliou, C.; Marshall, G.P.; Chen, W. (January 1989). "Light stabiliser, antioxidant and pigment interactions in the thermal and photochemical oxidation of polyethylene films". Polymer Degradation and Stability 24 (1): 17–31. doi:10.1016/0141-3910(89)90130-4.
- ↑ Pfaendner, Rudolf (December 2013). "(Photo)oxidative degradation and stabilization of flame retarded polymers". Polymer Degradation and Stability 98 (12): 2430–2435. doi:10.1016/j.polymdegradstab.2013.07.005.
- ↑ Ammala, Anne; Bateman, Stuart; Dean, Katherine; Petinakis, Eustathios; Sangwan, Parveen; Wong, Susan; Yuan, Qiang; Yu, Long et al. (August 2011). "An overview of degradable and biodegradable polyolefins". Progress in Polymer Science 36 (8): 1015–1049. doi:10.1016/j.progpolymsci.2010.12.002.
- ↑ "on the impact of the use of oxo-degradable plastic, including oxo-degradable plastic". EUROPEAN. https://ec.europa.eu/environment/circular-economy/pdf/oxo-plastics.pdf.
- ↑ Babetto, Alex S.; Antunes, Marcela C.; Bettini, Sílvia H. P.; Bonse, Baltus C. (February 2020). "A Recycling-Focused Assessment of the Oxidative Thermomechanical Degradation of HDPE Melt Containing Pro-oxidant". Journal of Polymers and the Environment 28 (2): 699–712. doi:10.1007/s10924-019-01641-6. https://repositorio.fei.edu.br/handle/FEI/3410.
- ↑ Aldas, Miguel; Paladines, Andrea; Valle, Vladimir; Pazmiño, Miguel; Quiroz, Francisco (2018). "Effect of the Prodegradant-Additive Plastics Incorporated on the Polyethylene Recycling". International Journal of Polymer Science 2018: 1–10. doi:10.1155/2018/2474176.
- ↑ the EU directive 2019/904 (Article 5), EU directive 5 June 2019
- ↑ L. Guedri-Knani, J. L. Gardette, M. Jacquet, A. Rivaton, Photoprotection of poly(ethylene-naphthalate) by zinc oxide coating, Surface and Coatings Technology, Volumes 180-181, 1 March 2004, Pages 71-75
- ↑ A. Moustaghfir, E. Tomasella, A. Rivaton, B. Mailhot, M. Jacquet, J. L. Gardette, J. Cellier, Sputtered zinc oxide coatings: structural study and application to the photoprotection of the polycarbonate, Surface and Coatings Technology, Volumes 180-181, 1 March 2004, Pages 642-645.
- ↑ Jacques, L.F.E (November 2000). "Accelerated and outdoor/natural exposure testing of coatings". Progress in Polymer Science 25 (9): 1337–1362. doi:10.1016/S0079-6700(00)00030-7.
- ↑ Kim, Sunwoo; Lee, Youngmin; Kim, Changhwan; Choi, Sunwoong (2022-01-17). "Analysis of Mechanical Property Degradation of Outdoor Weather-Exposed Polymers" (in en). Polymers 14 (2): 357. doi:10.3390/polym14020357. ISSN 2073-4360. PMID 35054761.
- ↑ Koriem, A.; Ollick, A. M.; Elhadary, M. (2021-08-01). "The effect of artificial weathering and hardening on mechanical properties of HDPE with and without UV stabilizers" (in en). Alexandria Engineering Journal 60 (4): 4167–4175. doi:10.1016/j.aej.2021.03.024. ISSN 1110-0168. https://www.sciencedirect.com/science/article/pii/S1110016821001800.
- ↑ 56.0 56.1 Lessa Belone, Maria Clara; Kokko, Marika; Sarlin, Essi (2022-09-01). "The effects of weathering-induced degradation of polymers in the microplastic study involving reduction of organic matter" (in en). Environmental Pollution 308: 119669. doi:10.1016/j.envpol.2022.119669. ISSN 0269-7491. PMID 35750308. https://www.sciencedirect.com/science/article/pii/S0269749122008831.
- ↑ Chamas, Ali; Moon, Hyunjin; Zheng, Jiajia; Qiu, Yang; Tabassum, Tarnuma; Jang, Jun Hee; Abu-Omar, Mahdi; Scott, Susannah L. et al. (2020-03-09). "Degradation Rates of Plastics in the Environment" (in en). ACS Sustainable Chemistry & Engineering 8 (9): 3494–3511. doi:10.1021/acssuschemeng.9b06635. ISSN 2168-0485. https://pubs.acs.org/doi/10.1021/acssuschemeng.9b06635.
- ↑ Celina, Mathew C.; Linde, Erik; Martinez, Estevan (March 2021). "Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation". Polymer Degradation and Stability 188: 109550. doi:10.1016/j.polymdegradstab.2021.109550.
 |
 KSF
KSF