Wurtzite crystal structure
Topic: Physics
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
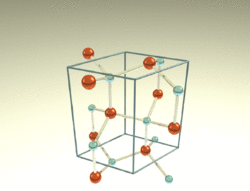
thumb|150px|right|General hexagonal crystal structure
The wurtzite crystal structure, named after the mineral wurtzite, is a crystal structure for various binary compounds. It is an example of a hexagonal crystal system. The chemical prototype is conventionally given as ZnS.[2]
Structure
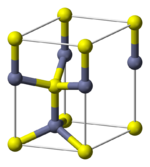
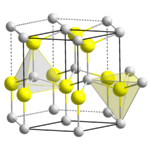
The wurtzite crystal structure is referred to by the Strukturbericht designation B4 and the Pearson symbol hP4. The corresponding space group is No. 186 (in International Union of Crystallography classification) or P63mc (in Hermann–Mauguin notation). The Hermann-Mauguin symbols in P63mc can be read as follows:[3]
- 63.. : a six fold screw rotation around the c-axis
- .m. : a mirror plane with normal {100}
- ..c : glide plane in the c-directions with normal {120}.
Among the compounds that can take the wurtzite structure are wurtzite itself (ZnS with up to 8% iron instead of zinc), silver iodide (AgI), zinc oxide (ZnO), cadmium sulfide (CdS), cadmium selenide (CdSe), silicon carbide (α-SiC), gallium nitride (GaN), aluminium nitride (AlN), boron nitride (w-BN) and other semiconductors.[4] In most of these compounds, wurtzite is not the favored form of the bulk crystal, but the structure can be favored in some nanocrystal forms of the material.
In materials with more than one crystal structure, the prefix "w-" is sometimes added to the empirical formula to denote the wurtzite crystal structure, as in w-BN.
Each of the two individual atom types forms a sublattice which is hexagonal close-packed (HCP-type). When viewed all together, the atomic positions are the same as in lonsdaleite (hexagonal diamond). Each atom is tetrahedrally coordinated.
The wurtzite structure is non-centrosymmetric (i.e., lacks inversion symmetry). Due to this, wurtzite crystals can (and generally do) have properties such as piezoelectricity and pyroelectricity, which centrosymmetric crystals lack.[citation needed]
References
- ↑ De Graef, Marc; McHenry, Michael E. (2012). Structure of Materials; An introduction to Crystallography, Diffraction and Symmetry. Cambridge University Press. p. 16. http://som.web.cmu.edu/StructuresAppendix2.pdf.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Hitchcock, Peter B (1988). International tables for crystallography volume A.. https://www.springer.com/series/6995.
- ↑ Togo, Atsushi; Chaput, Laurent; Tanaka, Isao (2015-03-20). "Distributions of phonon lifetimes in Brillouin zones". Physical Review B 91 (9): 094306. doi:10.1103/PhysRevB.91.094306.
 KSF
KSF