Alkane stereochemistry
 From Wikidoc - Reading time: 3 min
From Wikidoc - Reading time: 3 min
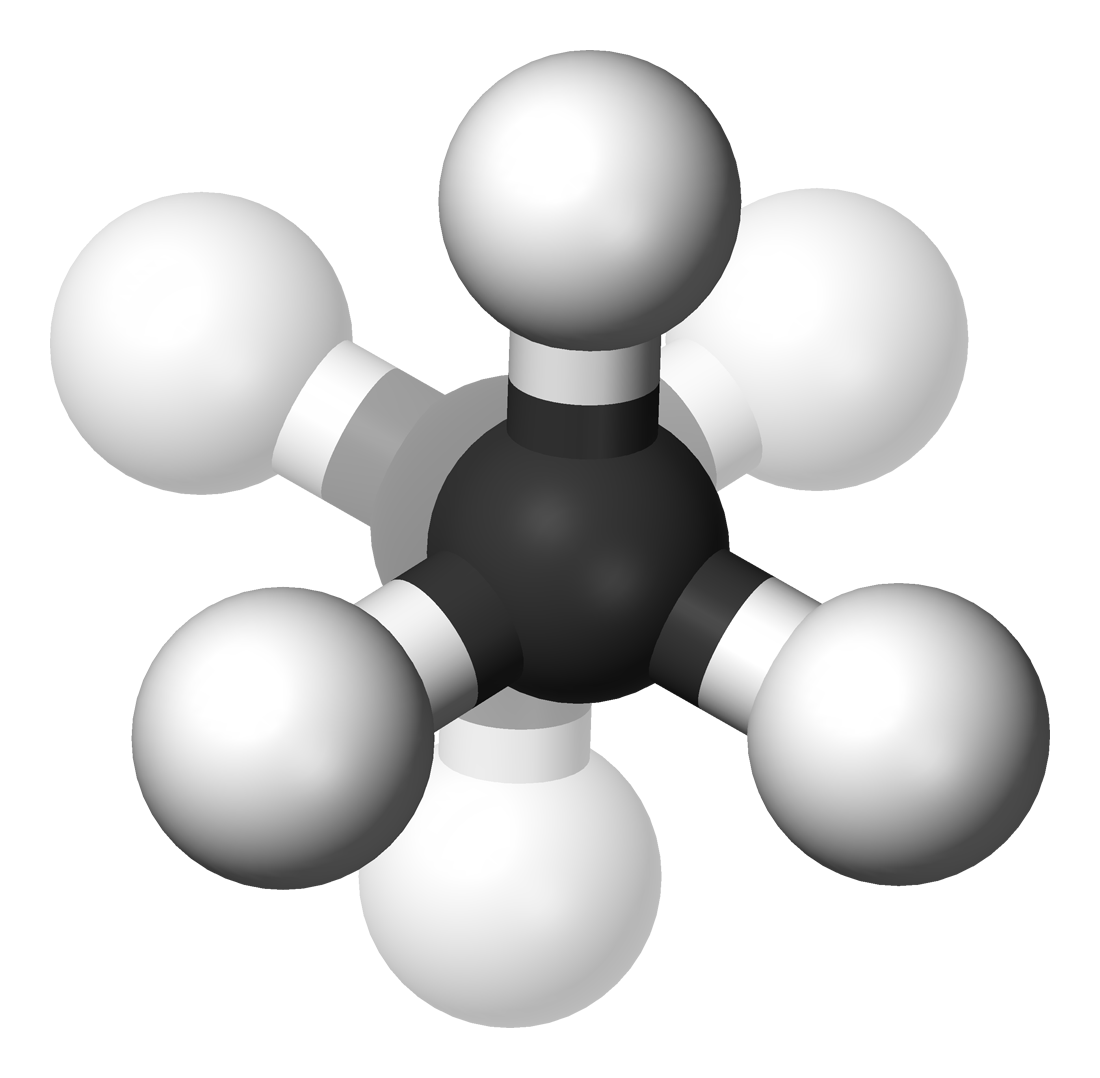
Alkane stereochemistry concerns the stereochemistry of linear alkanes and the linear alkane conformers. The existence of more than one conformation is due to hindered rotation around sp3 hybridised carbon carbon bonds. The smallest molecule with such a chemical bond, ethane, is found to exist as two conformers, staggered and eclipsed.
- A staggered conformation is a chemical conformation that exists in any open chain single chemical bond connecting two sp3 hybridised atoms as a conformational energy minimum.
- an eclipsed conformation is a chemical conformation that exists in any open chain single chemical bond connecting two sp3 hybridised atoms as a conformational energy maximum due to steric hindrance.
Conformations[edit | edit source]
Alkane stereochemistry concerns the stereochemistry of linear alkanes and the linear alkane conformers. The existence of more than one conformation is due to hindered rotation around sp3 hybridised carbon carbon bonds. The smallest molecule with such a chemical bond, ethane, exists as an infinite number of conformations with respect to rotation around the C-C bond; two of these are recognised as energy minimum (staggered) and energy maximum (eclipsed) forms. The importance of these is seen by extension of these concepts to more complex molecules for which stable conformations may be predicted as minimum energy forms.
In the example of staggered ethane in Newman projection a hydrogen atom on one carbon atom has a 60° torsional angle or torsion angle with respect to the nearest hydrogen atom on the other carbon so that steric hindrance is minimised. The staggered conformation is more stable by 12.5 kJ/mole than the eclipsed conformation which is the energy maximum for ethane. In the eclipsed conformation the torsional angle is minimized.
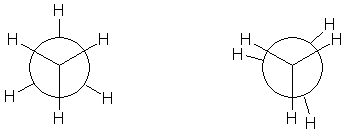

In butane, the two staggered conformations are no longer equivalent and represent two distinct conformers:the anti conformation (left-most, below) and the gauche conformation (right-most, below).
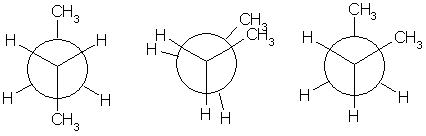
Both conformations are free of torsional strain but in the gauche conformation the two methyl groups are in closer proximity than the sum of their van der Waals radii. The interaction between the two methyl groups is repulsive (van der Waals strain) and an energy barrier results.
A measure of the potential energy stored in butane conformers with greater steric hindrance than the 'anti' conformer ground state is given by these values:
- Gauche, conformer - 3.8 kJ/mol
- Eclipsed H and CH3 - 16 kJ/mol
- Eclipsed CH3 and CH3 - 19 kJ/mol
The eclipsed methyl groups exert a greater steric strain because of their greater electron density compared to lone hydrogen atoms.
The textbook explanation for the existence of the energy maximum for an eclipsed conformation in ethane is steric hindrance but with a C-C bond length of 154 pm and a Van der Waals radius for hydrogen of 120 pm the hydrogen atoms in ethane are never in each other's way. This inconsistency is a topic of debate to this day. One alternative to the steric hindrance explanation is based on hyperconjugation.[1][2] In terms of molecular orbital theory in the staggered conformation one C-H sigma bonding orbital donates electrons to the antibonding orbital of the other C-H bond with maximum overlap of the same-sign lobes. This stabilizing overlap is not present in the eclipsed conformation.
Definitions[edit | edit source]
Many definitions exist that describe a specific conformation (IUPAC Gold Book):
- a torsion angle of ±60° is called gauche [3].
- a torsion angle between 0° and ± 90° is called syn (s),
- a torsion angle between ± 90° and 180° is called anti (a).
- a torsion angle between 30° and 150° or between –30° and –150° is called clinal
- a torsion angle between 0° and 30° or 150° and 180° is called periplanar (p)
- a torsion angle between 0° to 30° is called synperiplanar or syn- or cis-conformation (sp)
- a torsion angle between 30° to 90° and –30° to –90° is called synclinal or gauche or skew (sc);
- a torsion angle between 90° to 150°, and –90° to –150° is called anticlinal (ac);
- a torsion angle between ± 150° to 180° is called antiperiplanar or anti or trans (ap).
Any strain resulting from torsion is also called Pitzer Strain or eclipsing strain.
Special cases[edit | edit source]
In n-pentane the terminal methyl groups experience additional pentane interference.
Replacing hydrogen by fluorine in polytetrafluoroethylene changes the stereochemistry from the zigzag geometry to that of a helix due to electrostatic repulsion of the fluorine atoms in the 1,3 positions. Evidence for the helix structure in the crystalline state is derived from X-ray crystallography and from NMR spectroscopy and circular dichroism in solution.[4]
See also[edit | edit source]
- More alkane conformations exist in cyclic alkanes : see cyclohexane conformations.
- More on the impact of gauche interactions: see Gauche Effect.
External links[edit | edit source]
References[edit | edit source]
- ↑ Hyperconjugation not steric repulsion leads to the staggered structure of ethane Pophristic, V. & Goodman, L. Nature 411, 565–568 (2001)Abstract
- ↑ Chemistry: A new twist on molecular shape Frank Weinhold Nature 411, 539-541 (31 May 2001)
- ↑ Anslyn, Eric V. and Dougherty, Dennis A. Modern Physical Organic Chemistry. University Science (July 15, 2005), 1083 pp. ISBN-10: 1891389319
- ↑ Conformational Analysis of Chiral Helical Perfluoroalkyl Chains by VCD Kenji Monde, Nobuaki Miura, Mai Hashimoto, Tohru Taniguchi, and Tamotsu Inabe J. Am. Chem. Soc.; 2006; 128(18) pp 6000 - 6001; Graphical abstract
 KSF
KSF