Amphetamine detailed information
 From Wikidoc - Reading time: 12 min
From Wikidoc - Reading time: 12 min
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | (±)-alpha-methylbenzeneethanamine, alpha-methylphenethylamine, beta-phenyl-isopropylamine |
| Pregnancy category |
|
| Routes of administration | Oral, intravenous, vaporization, insufflation, suppository, sublingual |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral 20–25%; nasal 75%; rectal 95–99%; intravenous 100% |
| Protein binding | 15–40% |
| Metabolism | Hepatic (CYP2D6[1]) |
| Elimination half-life | 10 hours for d-isomer, 13 hours for l-isomer |
| Excretion | Renal; significant portion unaltered |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
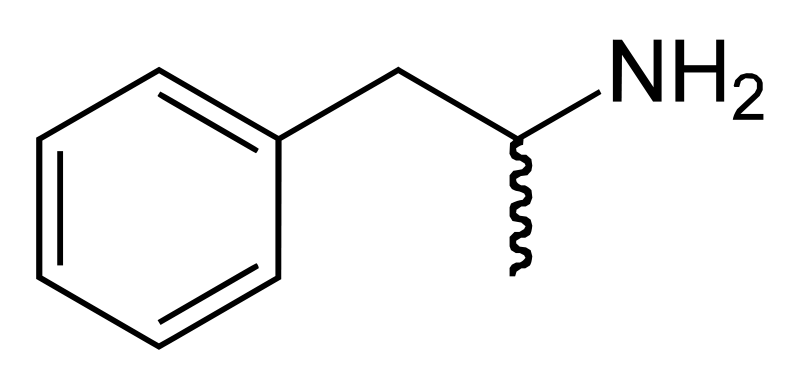
| Formula | C9H13N |
| Molar mass | 135.2084 |
| 3D model (JSmol) | |
| Melting point | 280 to 281 °C (Expression error: Unrecognized word "to". °F) |
| Solubility in water | 50–100 mg/mL (16C°) mg/mL (20 °C) |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Amphetamine is a prescription CNS stimulant commonly used to treat attention-deficit hyperactivity disorder (ADHD) in adults and children. It is also used to treat symptoms of traumatic brain injury and the daytime drowsiness symptoms of narcolepsy and chronic fatigue syndrome. Initially it was more popularly used to diminish the appetite and to control weight. Brand names of the drugs that contain amphetamine include Adderall and Dexedrine. The drug is also used illegally as a recreational club drug and as a performance enhancer. The name amphetamine is derived from its chemical name: alpha-methylphenethylamine. The name is also used to refer to the class of compounds derived from amphetamine, often referred to as the substituted amphetamines.
History[edit | edit source]
Amphetamine was first synthesized in 1887 by Lazar Edeleanu at the University of Berlin. He named the compound phenylisopropylamine. It was one of a series of compounds related to the plant derivative ephedrine, which had been isolated from Ma-Huang that same year by Nagayoshi Nagai. No pharmacological use was found for amphetamine until 1927, when pioneer psychopharmacologist Gordon Alles resynthesized it.[2][3] Alles was part of a group of researchers looking for an ephedrine substitute. In 1937, it became available in tablet form. During World War II it was extensively used to combat fatigue and increase alertness in soldiers. After decades of reported abuse, the FDA banned Benzedrine inhalers, and limited amphetamines to prescription use in 1965, but illegal use became common. Amphetamine became a schedule II drug with the passage of the Controlled Substances Act in 1970.
The related compound methamphetamine was first synthesized from ephedrine in Japan in 1918 by chemist Akira Ogata via reduction of ephedrine using red phosphorus and iodine. The German military was notorious for their use of methamphetamine in World War II. It is also known that Adolf Hitler was receiving daily shots of a medicine that contained certain essential vitamins and amphetamines. The German pharmaceutical Pervitin is an oral pill of 3 mg which was made available in 1938, but by mid-1941 it became a controlled substance, reportedly because of the amount of time needed for a soldier to rest and recover after use. Military doctors were then given guidelines on how they should issue it.
In 1997[4] and 1998,[5] researchers at Texas A&M University reported finding amphetamine and methamphetamine in the foliage of two Acacia species native to Texas, A. berlandieri and A. rigidula. Previously, both of these compounds had been thought to be human inventions.[6]
Indications[edit | edit source]
Indicated for:
|
Contraindications:
|
| Side effects:
Eye:
|
Along with methylphenidate (Ritalin, Concerta, etc.), amphetamine is one of the standard treatments for ADHD. Beneficial effects for ADHD can include improved impulse control, improved concentration, decreased sensory overstimulation, decreased irritability and decreased anxiety. These effects can be dramatic in both young children and adults. The ADHD medication Adderall is composed of four different amphetamine salts, and Adderall XR is a timed-release formulation of these same salt forms.
When used within the recommended doses, side-effects like loss of appetite tend to decrease over time. However, amphetamines last longer in the body than methylphenidate (Ritalin, Concerta, etc.), and tend to have stronger side-effects on appetite and sleep.
Amphetamines are also a standard treatment for narcolepsy, as well as other sleeping disorders. They are generally effective over long periods of time without producing addiction or physical dependence.
Amphetamines are sometimes used to augment anti-depressant therapy in treatment-resistant depression.
Medical use for weight loss is still approved in some countries, but is regarded as obsolete and dangerous in others.
Contraindications[edit | edit source]
Stimulants such as amphetamines elevate cardiac output and blood pressure making them dangerous for use by patients with a history of heart disease or hypertension. Also, patients with a history of drug dependence or anorexia should not be treated with amphetamines due to their addictive and appetite suppressing properties. Amphetamines can cause a life-threatening complication in patients taking MAOI antidepressants. Amphetamine is not suitable for patients with a history of glaucoma.
Amphetamines have also been shown to pass through into breast milk. Because of this, mothers taking medications containing amphetamines are advised to avoid breastfeeding during their course of treatment.[7]
Pharmacology[edit | edit source]
Chemical Properties[edit | edit source]
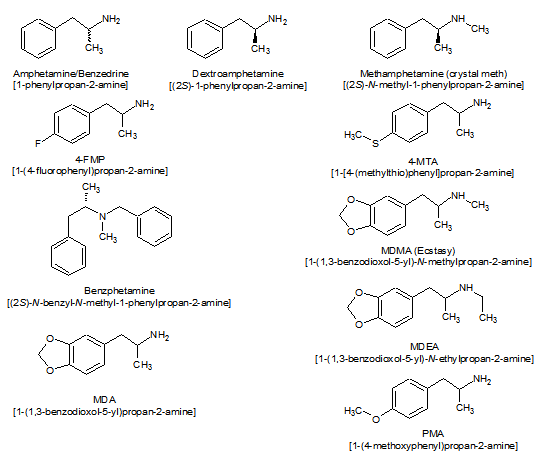
Amphetamine is a chiral compound. The racemic mixture can be divided into its optical antipodes: levo- and dextro-amphetamine. Amphetamine is the parent compound of its own structural class, comprising a broad range of psychoactive derivatives, e.g., MDMA (Ecstasy) and the N-methylated form, methamphetamine. Amphetamine is a homologue of phenethylamine.
At first, the medical drug came as the salt racemic-amphetamine sulfate (racemic-amphetamine contains both isomers in equal amounts). Today, dextroamphetamine sulfate is the predominant form of the drug used;[citation needed] it consists entirely of the d-isomer. Attention disorders are often treated using Adderall or a generic equivalent, a formulation of mixed amphetamine salts that contain both racemic-amphetamine and d-amphetamine in the sulfate and saccharate forms mixed to a final ratio of 3 parts d-amphetamine to 1 part l-amphetamine.
Pharmacodynamics[edit | edit source]
Amphetamine has been shown to both diffuse through the cell membrane and travel via the dopamine transporter (DAT) to increase concentrations of dopamine in the neuronal terminal.
Amphetamine, both as d-amphetamine (dextroamphetamine) and l-amphetamine (or a racemic mixture of the two isomers), is believed to exert its effects by binding to the monoamine transporters and increasing extracellular levels of the biogenic amines dopamine, norepinephrine (noradrenaline) and serotonin. It is hypothesized that d-amphetamine acts primarily on the dopaminergic systems, while l-amphetamine is comparatively norepinephrinergic (noradrenergic). The primary reinforcing and behavioral-stimulant effects of amphetamine, however, are linked to enhanced dopaminergic activity, primarily in the mesolimbic dopamine system.
Amphetamine and other amphetamine-type stimulants principally act to release dopamine into the synaptic cleft. The increased amphetamine concentration releases endogenous stores of dopamine from vesicular monoamine transporters (VMATs), thereby increasing intra-neuronal concentrations of transmitter. This increase in concentration effectively reverses transport of dopamine via the dopamine transporter (DAT) into the synapse.[8] In addition, amphetamine binds reversibly to the DATs and blocks the transporter's ability to clear DA from the synaptic space. Amphetamine also acts in this way with norepinephrine (noradrenaline) and to a lesser extent serotonin.
In addition, amphetamine binds to a group of receptors called TrAce Amine Receptors (TAAR).[9] TAAR are a newly discovered receptor system which seems to be affected by a range of amphetamine-like substances called trace amines.
Physical effects[edit | edit source]
- Short-term physiological effects vary greatly, depending on dosage used and the method in which the drug is taken. At therapeutic levels, the most common effects are decreased appetite, increased stamina, and physical energy.[citation needed].
- Abuse or overdose effects[citation needed] can include tremor, restlessness, changed sleep patterns, anxiety hyperhidrosis,psychomotor agitation, nausea, tachycardia, irregular heart rate, hypertension, headaches, hyperreflexia, tachypnea, gastrointestinal narrowing, and weakened immune system and increase in pre-existing anxiety and poor skin condition. [citation needed]Fatigue and depression can follow the excitement stage. Erectile dysfunction, heart problems, stroke, and liver, kidney and lung damage can result from prolonged abuse [citation needed]. When insufflated, amphetamine can lead to a deterioration of the lining of the nostrils[citation needed]. Overdose can be treated with chlorpromazine.[10]
Psychological effects[edit | edit source]
- Short-term psychological effects of the drug at therapeutic levels could include alertness, euphoria, increased concentration, rapid talking, increased confidence, and increased social responsiveness [citation needed]. Effects of the drug when abused could include, hallucinations, and loss of REM sleep the night after use [citation needed].
- Long-term amphetamine abuse can induce psychological effects that include insomnia, mental states resembling schizophrenia, aggressiveness (not associated with schizophrenia), addiction or dependence with accompanying withdrawal symptoms, irritability, confusion, and panic [citation needed]. Chronic and/or extensively-continuous use can lead to amphetamine psychosis, which causes delusions and paranoia, but this is uncommon when taken as prescribed. The abuse of an amphetamine is highly addictive, and, with chronic abuse, tolerance develops very quickly. Withdrawal, although not physiologically threatening, is an unpleasant experience (including paranoia, depression, difficult breathing, dysphoria, gastric fluctuations and/or pain, and lethargia) [citation needed]. This commonly leads chronic users to re-dose amphetamine frequently, explaining tolerance and increasing the possibility of addiction.
Dependence & Addiction[edit | edit source]
Tolerance is developed rapidly in amphetamine abuse, therefore increasing the amount of the drug that is needed to satisfy the addiction.[11] Repeated amphetamine use can produce "reverse tolerance", or sensitization to some psychological effects.[12][13][14][15][16] Many abusers will repeat the amphetamine cycle by taking more of the drug during the withdrawal. This leads to a very dangerous cycle and may involve the use of other drugs to get over the withdrawal process. Abusers will commonly stay up for 2 or 3 days avoiding the withdrawals then dose themselves with benzodiazepines or barbiturates to help them stay calm while they recuperate. The constant switching from uppers to downers can cause serious damage to the CNS and brain [citation needed]. Chronic abusers of amphetamines typically snort or resort to drug injection to experience the full effects of the drug in a faster and more intense way, with the added risks of infection, vein damage, and higher risk of overdose. Because of the abuse of amphetamines in the U.S., most brands were discontinued by the 1990s, including the highly abused brand names Biphetamine (known as "black beauties") and Preludin, known on the street as "slams", whose coating was peeled and then injected. Only a few brands of amphetamines are still produced in the United States: those prescribed for narcolepsy, attention-deficit hyperactivity disorder, treatment-resistant depression, and extreme obesity.[citation needed]
Performance-enhancing use[edit | edit source]
Amphetamine is used by college and high-school students as a study and test-taking aid.[17] Amphetamine increases energy levels, concentration, and motivation, allowing students to study for an extended period of time. These drugs are often acquired through ADHD prescriptions to students and peers, rather than illicitly produced drugs. [18]
Amphetamine is also used by professional,[19] collegiate[20] and high school[21] athletes for its strong stimulant effect. Energy levels are perceived to be dramatically increased and sustained, believed to allow for more vigorous and longer play, though at least one study has found that this effect is not measurable.[22] This practice can be extremely dangerous, and athletes have died as a result, for example, British cyclist Tom Simpson. A doctor (the same doctor who prescribed 800 children Adderall in order to lose weight) has created a diet based around amphetamines, called the adderall diet. CNN ran a story about the diet and the doctor and people expressed outrage. [citation needed]
Amphetamine use has historically been especially common among Major League Baseball (MLB) athletes and is usually known by the slang term "greenies".[23] In 2006, MLB banned the use of amphetamines and the ban is enforced by periodic drug-testing. Consequences if a player tests positive are significant, but MLB has received some criticism because these consequences are dramatically less severe than for steroids, with the first offense bringing only a warning and further testing[24].[25][26]
Truck drivers, especially long-haul drivers, take amphetamine[27] to combat symptoms of somnolence and to increase their concentration on driving.
Legal issues[edit | edit source]
- In the United Kingdom, amphetamines were regarded as Class B drugs. The maximum penalty for unauthorised possession is five years in prison and an unlimited fine. The maximum penalty for illegal supply is fourteen years in prison and an unlimited fine. Methamphetamine has recently been reclassified to Class A, penalties for possession of which are more severe (7 years in prison and an unlimited fine).[28]
- In the Netherlands, amphetamine and methamphetamine are List I drugs of the Opium Law, but the dextro isomer of amphetamine is indicated for ADD/ADHD and narcolepsy and available for prescription as 5 and 10 mg generic tablets, and 5 and 10 mg gelcapsules.
- In the United States, amphetamine and methamphetamine are Schedule II drugs, classified as CNS (Central Nervous System) Stimulants.[29] A Schedule II drug is classified as one that has a high potential for abuse, has a currently-accepted medical use and is used under severe restrictions, and has a high possibility of severe psychological and physiological dependence.
Internationally, amphetamine is a Schedule II drug under the Convention on Psychotropic Substances.[30]
Books[edit | edit source]
- Seabrook, Jeremy (1996). In the Cities of the South: scenes from a developing world. London; New York: Verso. ISBN 1-85984-986-5.
See also[edit | edit source]
- Adderall
- Attention Deficit Hyperactivity Disorder
- Benzylpiperazine
- Clandestine chemistry
- Ethylamphetamine
- Dextroamphetamine (Dexedrine)
- Lisdexamfetamine (Vyvanse)
- Methamphetamine (Desoxyn)
- Methylphenidate (Ritalin, Concerta)
- Phenethylamines
- Propylamphetamine
- Psychostimulants
References and notes[edit | edit source]
- ↑ Determination of amphetamine, methamphetamine, and ...
- ↑ Shulgin, Alexander (1992). "6 – MMDA". PiHKAL. Berkeley, California: Transform Press. p. 39. ISBN 0-9630096-0-5. Unknown parameter
|coauthors=ignored (help) - ↑ "meth – Anatomy of a designer drug". Massey University Alumni Magazine (18): 12. 2005. Retrieved 2007-06-23.
But amphetamine's pharmacological potential was missed, and the molecule lapsed into obscurity until 1927, when it was resynthesised by Gordon Alles, one of a group of chemists looking for an ephedrine substitute. Alles also prepared amphetamine in a volatile form. In 1932 it became available under the brand name Benzedrine as an over-the-counter inhaler to treat respiratory problems – and the contents were widely used for purposes other than the treatment of respiratory disorders.
Unknown parameter|month=ignored (help) - ↑ Clement, Beverly A., Goff, Erik Allen Burt, Christina M. and Forbes, T. David A. (1997). Toxic amines and alkaloids from Acacia berlandieri. Phytochemistry 46(2), pp 249-254
- ↑ Clement, Beverly A., Goff, Christina M. and Forbes, T. David A. (1998). Toxic amines and alkaloids from Acacia rigidula. Phytochemistry 49(5), pp 1377-1380
- ↑ Ask Dr. Shulgin Online: Acacias and Natural Amphetamine
- ↑ [1] FDA PDF 2004
- ↑ Sulzer, D., (2005). Mechanisms of neurotransmitter release by amphetamines: A review. Progress in Neurobiology, 75(6);406-433.
- ↑ jpet.aspetjournals.org Research published in the Journal of Pharmacology And Experimental Therapeutics (2007)
- ↑ rxlist.com
- ↑ "Amphetamines: Drug Use and Abuse: Merck Manual Home Edition" (html). Merck. Unknown parameter
|accessyear=ignored (|access-date=suggested) (help); Unknown parameter|accessmonthday=ignored (help) - ↑ Leith N, Kuczenski R (1981). "Chronic amphetamine: tolerance and reverse tolerance reflect different behavioral actions of the drug". Pharmacol Biochem Behav. 15 (3): 399–404. PMID 7291243.
- ↑ Chaudhry I, Turkanis S, Karler R (1988). "Characteristics of "reverse tolerance" to amphetamine-induced locomotor stimulation in mice". Neuropharmacology. 27 (8): 777–81. PMID 3216957.
- ↑ Chronic Amphetamine Use and Abuse
- ↑ Sax KW, Strakowski SM (2001). "Behavioral sensitization in humans". J Addict Dis. 20 (3): 55–65. PMID 11681593.
- ↑ I. Boileau, A. Dagher, M. Leyton, R. N. Gunn, G. B. Baker, M. Diksic and C. Benkelfat (2006). "Modeling Sensitization to Stimulants in Humans: An [11C]Raclopride/Positron Emission Tomography Study in Healthy Men". Arch Gen Psychiatry. 63 (12): 1386–1395.
- ↑ Twohey, Megan (2006-03-25). "Pills become an addictive study aid". JS Online. Retrieved 2007-12-02.
- ↑ The Illicit Market for ADHD Prescription Drugs in Queensland (PDF), Queensland Crime and Misconduct Commission, April 2002, retrieved 13 January 2008
- ↑ Yesalis, Charles E. (2005-12). "Anabolic Steroid and Stimulant Use in North American Sport between 1850 and 1980". Sport in History. 25 (3): 434–451. Retrieved 2007-12-02. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Template:Cite
- ↑ Template:Cite
- ↑ Margaria, R (1964-07-01). "The effect of some drugs on the maximal capacity of athletic performance in man". European Journal of Applied Physiology. 20 (4): 281–287. doi:10.1007/BF00697020. Retrieved 2007-12-02. Unknown parameter
|coauthors=ignored (help) - ↑ Frias, Carlos (2006-04-02). "Baseball and amphetamines". Palm Beach Post. Retrieved 2007-12-02.
- ↑ Kreidler, Mark (2005-11-15). "Baseball finally brings amphetamines into light of day". ESPN.com. Retrieved 2007-12-02.
- ↑ Klobuchar, Jim (2006-03-31). "Can baseball make a clean sweep?". Christian Science Monitor. Retrieved 2007-12-02.
- ↑ Associated Press (2007-01-18). "MLB owners won't crack down on 'greenies'". MSNBC.com. Retrieved 2007-12-02.
- ↑ Lund, Adrian K (1989). "Drug Use by Tractor-Trailer Drivers" (PDF). In Steven W. Gust (ed.). Drugs in the Workplace: Research and Evaluation Data. National Institute on Drug Abuse Research. Rockville, MD: National Institute on Drug Abuse. pp. 47–67. Retrieved 2007-12-02.
This study has provided the first objective data regarding the use of potentially abusive drugs by tractor-trailer drivers... Prescription stimulants, such as amphetamine, methamphetamine, and phentermine were found in 5 percent of the [317] drivers [who participated in the study], often in combination with similar but less potent stimulants, such as phenylpropanolamine. Nonprescription stimulants were detected in 12 percent of the drivers, about half of whom gave no medical explanation for their presence... One limitation of these findings is that 12 percent of the randomly selected drivers refused to participate in the study or provided insufficient urine and blood for testing; the distribution of drugs among these 42 drivers is unknown... Finally, the results apply to tractor-trailer drivers operating on a major east-west interstate route in Tennessee. Drug incidence among other truck-driver populations are unknown and may be higher or lower than reported here. (64)
Unknown parameter|coauthors=ignored (help) - ↑ Template:Cite web title=homeoffice url=http://www.homeoffice.gov.uk/drugs/drugs-law/Class-a-b-c/ accessdate=2007-07-23
- ↑ "Trends in Methamphetamine/Amphetamine Admissions to Treatment: 1993-2003" (html). Substance Abuse and Mental Health Services Administration. Unknown parameter
|accessyear=ignored (|access-date=suggested) (help); Unknown parameter|accessmonthday=ignored (help) - ↑ "List of psychotropic substances under international control" (PDF). International Narcotics Control Board. Unknown parameter
|accessyear=ignored (|access-date=suggested) (help); Unknown parameter|accessmonthday=ignored (help)
External links[edit | edit source]
- Adderall addiction and treatment
- Template:PubChemLink (D-form — dextroamphetamine)
- Template:PubChemLink (L-form and D, L-forms)
- Template:PubChemLink (L-form — Levamphetamine or L-amphetamine)
- List of 504 Compounds Similar to Amphetamine (PubChem)
- EMCDDA drugs profile: Amphetamine (2007)
- Drugs.com - Amphetamine
- Asia & Pacific Amphetamine-Type Stimulants Information Centre
- Erowid Amphetamine (Adderall) Vault
Template:Phenethylamines Template:Amphetamines Template:Psychostimulants, agents used for ADHD and nootropics Template:Stimulants Template:Methamphetamine
ar:أمفيتامين bg:Амфетамин cs:Amfetamin da:Amfetamin de:Amphetamin et:Amfetamiin el:Αμφεταμίνη gl:Anfetamina hr:Amfetamin id:Amfetamin it:Anfetamina he:אמפטמין lt:Amfetaminas hu:Amfetamin ms:Amfetamina nl:Amfetamine no:Amfetamin nn:Amfetamin sl:Amfetamin sr:Амфетамин fi:Amfetamiini sv:Amfetamin uk:Амфетамін
 KSF
KSF