Antibiotic
 From Wikidoc - Reading time: 12 min
From Wikidoc - Reading time: 12 min
|
WikiDoc Resources for Antibiotic |
|
Articles |
|---|
|
Most recent articles on Antibiotic |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Antibiotic at Clinical Trials.gov Clinical Trials on Antibiotic at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Antibiotic
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Antibiotic Discussion groups on Antibiotic Patient Handouts on Antibiotic Directions to Hospitals Treating Antibiotic Risk calculators and risk factors for Antibiotic
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Antibiotic |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
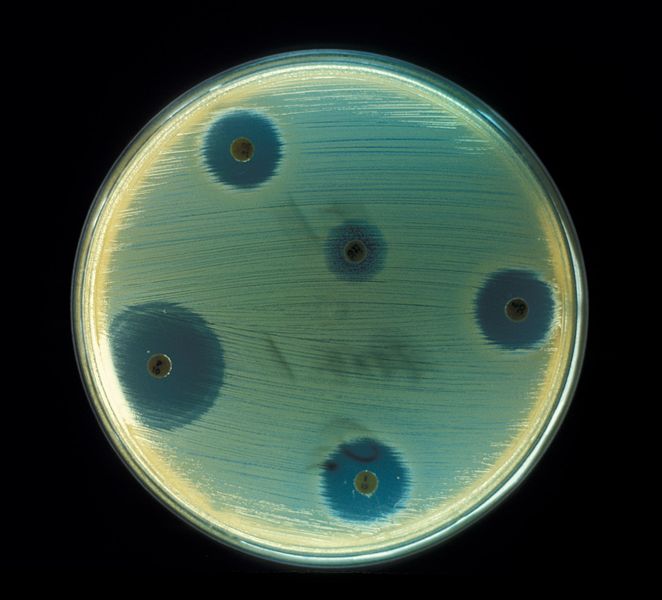
Overview[edit | edit source]
An antibiotic is a chemical compound that inhibits or abolishes the growth of microorganisms, such as bacteria, fungi, or protozoans. The term originally referred to any agent with biological activity against living organisms; however, "antibiotic" now is used to refer to substances with anti-bacterial, anti-fungal, or anti-parasitical activity. The first antibiotic compounds used in modern medicine were produced and isolated from living organisms, such as the penicillin class produced by fungi in the genus Penicillium, or streptomycin from bacteria of the genus Streptomyces. With advances in organic chemistry many antibiotics are now also obtained by chemical synthesis, such as the sulfa drugs. Many antibiotics are relatively small molecules with a molecular weight less than 2000 Da.
Unlike previous treatments for infections, which often consisted of administering chemical compounds such as strychnine and arsenic, with high toxicity also against mammals, antibiotics from microbes had no or few side effects and high effective target activity. Most anti-bacterial antibiotics do not have activity against viruses, fungi, or other microbes. Anti-bacterial antibiotics can be categorized based on their target specificity: "narrow-spectrum" antibiotics target particular types of bacteria, such as Gram-negative or Gram-positive bacteria, while broad-spectrum antibiotics affect a wide range of bacteria.
The effectiveness of individual antibiotics varies with the location of the infection, the ability of the antibiotic to reach the site of infection, and the ability of the microbe to inactivate or excrete the antibiotic. Some anti-bacterial antibiotics destroy bacteria (bactericidal), whereas others prevent bacteria from multiplying (bacteriostatic).
Oral antibiotics are simply ingested, while intravenous antibiotics are used in more serious cases, such as deep-seated systemic infections. Antibiotics may also sometimes be administered topically, as with eye drops or ointments.
In the last few years, three new classes of antibiotics have been brought into clinical use. This follows a 40-year hiatus in discovering new classes of antibiotic compounds. These new antibiotics are of the following three classes: cyclic lipopeptides (daptomycin), glycylcyclines (tigecycline), and oxazolidinones (linezolid). Tigecycline is a broad-spectrum antibiotic, while the two others are used for gram-positive infections. These developments show promise as a means to counteract the growing bacterial resistance to existing antibiotics.
History[edit | edit source]
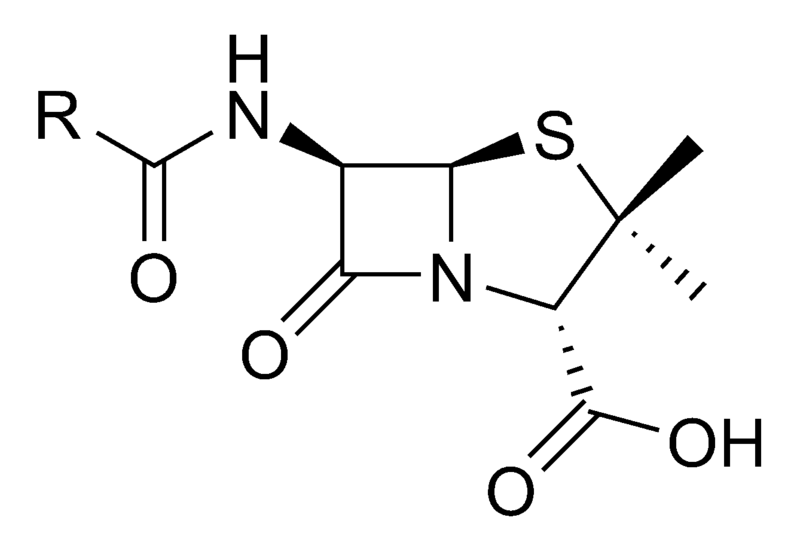
Although potent antibiotic compounds for treatment of human diseases caused by bacteria (such as tuberculosis, bubonic plague, or leprosy) were not isolated and identified until the twentieth century, the first known use of antibiotics was by the ancient Chinese over 2,500 years ago.[1] Many other ancient cultures, including the ancient Egyptians and ancient Greeks already used molds and plants to treat infections, owing to the production of antibiotic substances by these organisms. At that time, however, the compounds having antibiotic activity and present in moulds or plants were unknown.
The antibiotic properties of Penicillium sp. were first described in France by Ernest Duchesne in 1897. However, his work went by without much notice from the scientific community until Alexander Fleming's discovery of Penicillin (see below).
Modern research on antibiotic therapy began in Germany with the development of the narrow-spectrum antibiotic Salvarsan by Paul Ehrlich in 1909, for the first time allowing an efficient treatment of the then-widespread problem of Syphilis. The drug, which was also effective against other spirochaetal infections, is no longer in use in modern medicine.
Antibiotics were further developed in Britain following the re-discovery of Penicillin in 1928 by Alexander Fleming. More than ten years later, Ernst Chain and Howard Florey became interested in his work, and came up with the purified form of penicillin. The three shared the 1945 Nobel Prize in Medicine. "Antibiotic" was originally used to refer only to substances extracted from a fungus or other microorganism, but has come to include also the many synthetic and semi-synthetic drugs that have antibacterial effects.
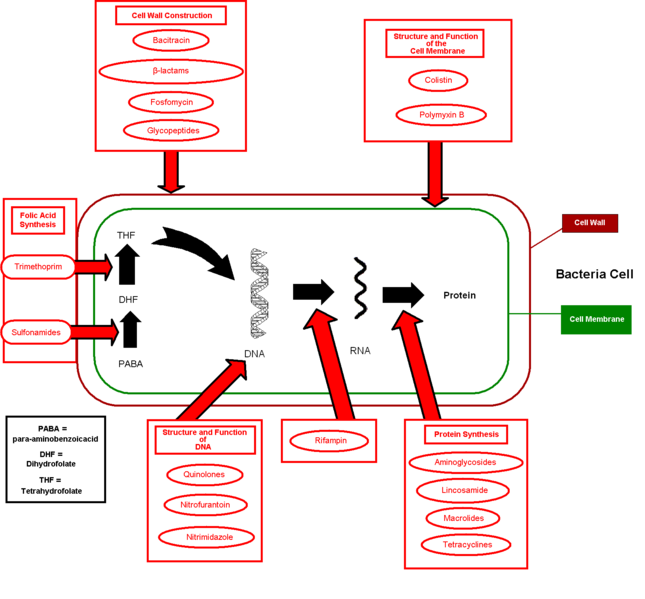
Classes of antibiotics[edit | edit source]
At the highest level, antibiotics can be classified as either bactericidal or bacteriostatic. Bactericidals kill bacteria directly where bacteriostatics prevent them from dividing. However, these classifications are based on laboratory behavior; in practice, both of these are capable of ending a bacterial infection.
| Generic Name | Brand Names | Common Uses | Side Effects | |
|---|---|---|---|---|
| Aminoglycosides | ||||
| Amikacin | Amikin | Infections caused by Gram-negative bacteria, such as Escherichia coli and Klebsiella particularly Pseudomonas aeruginosa. Effective against Aerobic bacteria (not obligate/facultative anaerobes. | ||
| Gentamicin | Garamycin | |||
| Kanamycin | Kantrex | |||
| Neomycin | ||||
| Netilmicin | Netromycin | |||
| Streptomycin | ||||
| Tobramycin | Nebcin | |||
| Paromomycin | Humatin | |||
| Ansamycins | ||||
| Geldanamycin | Experimental, as antitumor antibiotics | |||
| Herbimycin | ||||
| Carbacephem | ||||
| Loracarbef | Lorabid | |||
| Carbapenems | ||||
| Ertapenem | Invanz | Bactericidal for both Gram positive and Gram negative organisms via inhibition of cell wall synthesis and therefore useful for empiric broad-spectrum antibacterial coverage. (Note MRSA resistance to this class.) |
| |
| Imipenem/Cilastatin | Primaxin | |||
| Meropenem | Merrem | |||
| Cephalosporins (First generation) | ||||
| Cefadroxil | Duricef |
| ||
| Cefazolin | Ancef | |||
| Cefalotin or Cefalothin | Keflin | |||
| Cephalexin | Keflex | |||
| Cephalosporins (Second generation) | ||||
| Cefaclor | Ceclor |
| ||
| Cefamandole | Mandole | |||
| Cefoxitin | Mefoxin | |||
| Cefprozil | Cefzil | |||
| Cefuroxime | Ceftin | |||
| Cephalosporins (Third generation) | ||||
| Cefixime | Suprax |
| ||
| Cefdinir | Omnicef | |||
| Cefditoren | Spectracef | |||
| Cefoperazone | Cefobid | |||
| Cefotaxime | Claforan | |||
| Cefpodoxime | ||||
| Ceftazidime | Fortaz | |||
| Ceftibuten | ||||
| Ceftizoxime | ||||
| Ceftriaxone | Rocephin | |||
| Cefdinir | ||||
| Cephalosporins (Fourth generation) | ||||
| Cefepime | Maxipime |
| ||
| Glycopeptides | ||||
| Teicoplanin | ||||
| Vancomycin | Vancocin | |||
| Macrolides | ||||
| Azithromycin | Zithromax, Sumamed, Zitrocin | Streptococcal infections, syphilis, respiratory infections, mycoplasmal infections, Lyme disease |
| |
| Clarithromycin | Biaxin | |||
| Dirithromycin | ||||
| Erythromycin | ||||
| Roxithromycin | ||||
| Troleandomycin | ||||
| Telithromycin | Ketek | Pneumonia | Visual Disturbance, LIVER TOXICITY. This medication's approval in the U.S. was controversial, and one doctor went to jail in followup attempts to ascertain its safety because she falsified the results of her part of the testing precisely because it seemed to cause liver problems, including liver failure, to a greater extent than would be expected of a common-use antibiotic. | |
| Spectinomycin | Antimetabolite, Anticancer | |||
| Monobactams | ||||
| Aztreonam | ||||
| Penicillins | ||||
| Amoxicillin | Novamox | Wide range of infections; penicillin used for streptococcal infections, syphilis, and Lyme disease |
| |
| Ampicillin | ||||
| Azlocillin | ||||
| Carbenicillin | ||||
| Cloxacillin | ||||
| Dicloxacillin | ||||
| Flucloxacillin | ||||
| Mezlocillin | ||||
| Nafcillin | ||||
| Penicillin | ||||
| Piperacillin | ||||
| Ticarcillin | ||||
| Polypeptides | ||||
| Bacitracin | Eye, ear or bladder infections; usually applied directly to the eye or inhaled into the lungs; rarely given by injection | Kidney and nerve damage (when given by injection) | ||
| Colistin | ||||
| Polymyxin B | ||||
| Quinolones | ||||
| Ciprofloxacin | Ciproxin, Ciplox | Urinary tract infections, bacterial prostatitis, bacterial diarrhea, gonorrhea | Nausea (rare), tendinosis (rare) | |
| Enoxacin | ||||
| Gatifloxacin | Tequin | |||
| Levofloxacin | Levaquin | |||
| Lomefloxacin | ||||
| Moxifloxacin | Avelox | |||
| Norfloxacin | ||||
| Ofloxacin | Ocuflox | |||
| Trovafloxacin | Trovan | |||
| Sulfonamides | ||||
| Mafenide | Urinary tract infections (except sulfacetamide and mafenide); mafenide is used topically for burns |
| ||
| Prontosil (archaic) | ||||
| Sulfacetamide | ||||
| Sulfamethizole | ||||
| Sulfanilimide (archaic) | ||||
| Sulfasalazine | ||||
| Sulfisoxazole | ||||
| Trimethoprim | ||||
| Trimethoprim-Sulfamethoxazole (Co-trimoxazole) (TMP-SMX) | Bactrim | |||
| Tetracyclines | ||||
| Demeclocycline | Syphilis, chlamydial infections, Lyme disease, mycoplasmal infections, acne rickettsial infections |
| ||
| Doxycycline | Vibramycin | |||
| Minocycline | Minocin | |||
| Oxytetracycline | Terracin | |||
| Tetracycline | Sumycin | |||
| Others | ||||
| Arsphenamine | Salvarsan | Spirochaetal infections (obsolete) | ||
| Chloramphenicol | Chloromycetin | |||
| Clindamycin | Cleocin | acne infections, prophylaxis before surgery | ||
| Lincoamycin | acne infections, prophylaxis before surgery | |||
| Ethambutol | Antituberculosis | |||
| Fosfomycin | ||||
| Fusidic acid | Fucidin | |||
| Furazolidone | ||||
| Isoniazid | Antituberculosis | |||
| Linezolid | Zyvox | |||
| Metronidazole | Flagyl | Giardia | ||
| Mupirocin | Bactroban | |||
| Nitrofurantoin | Macrodantin, Macrobid | |||
| Platensimycin | ||||
| Pyrazinamide | Antituberculosis | |||
| Quinupristin/Dalfopristin | Syncercid | |||
| Rifampin or Rifampicin | Binds to the β subunit of "RNA polymerase" to inhibit transcription of mostly "Gram positive" and "mycobacteria" | Reddish-orange sweat, tears, and urine | ||
| Tinidazole | ||||
| Generic Name | Brand Names | Common Uses | Side Effects | |
Production[edit | edit source]
Since the first pioneering efforts of Florey and Chain in 1939, the importance of antibiotics to medicine has led to much research into discovering and producing them. The process of production usually involves screening of wide ranges of microorganisms, testing and modification. Production is carried out using fermentation; a process that is important in anaerobic conditions when there is no oxidative phosphorylation to maintain the production of adenosine triphosphate (ATP) by glycolysis.
Side effects[edit | edit source]
Possible side effects are varied, depend on the antibiotics used and the microbial organisms targeted. Adverse effects can range from fever and nausea to major allergic reactions including photodermatitis. One of the more common side effects is diarrhea, sometimes caused by the anaerobic bacterium Clostridium difficile, which results from the antibiotic disrupting the normal balance of the intestinal flora,[3] Such overgrowth of pathogenic bacteria may be alleviated by ingesting probiotics during a course of antibiotics. An antibiotic-induced disruption of the population of the bacteria normally present as constituents of the normal vaginal flora may also occur, and may lead to overgrowth of yeast species of the genus Candida in the vulvo-vaginal area. [4] Other side effects can result from interaction with other drugs, such as elevated risk of tendon damage from administration of a quinolone antibiotic with a systemic corticosteroid.
It is a common assertion that some antibiotics can interfere with the efficiency of birth control pills. Although there remain few known cases of complication, the majority of antibiotics do not interfere with contraception, despite widespread misinformation to the contrary.[5]
Antibiotic misuse[edit | edit source]
Common forms of antibiotic misuse include failure to take the entire prescribed course of the antibiotic, or failure to rest for sufficient recovery allowing clearance from the infecting organism. These practices may cause the development of bacterial populations with antibiotic resistance. Inappropriate antibiotic treatment is another common form of antibiotic misuse. A common example is the use of antibacterial antibiotics to treat viral infections such as the common cold.
Animals[edit | edit source]
It is estimated that greater than 50% of the antibiotics used in U.S. are given to feed animals (e.g. chickens, pigs and cattle) in the absence of disease.[6] Antibiotic use in food animal production has been associated with the emergence of antibiotic-resistant strains of bacteria including Salmonella spp., Campylobacter spp., Escherichia coli, and Enterococcus spp. Evidence from some US and European studies suggest that these resistant bacteria cause infections in humans that do not respond to commonly prescribed antibiotics. In response to these practices and attendant problems, several organizations (e.g. The American Society for Microbiology (ASM), American Public Health Association (APHA) and the American Medical Association (AMA)) have called for restrictions on antibiotic use in food animal production and an end to all non-therapeutic uses. However, delays in regulatory and legislative actions to limit the use of antibiotics are common, and may include resistance to these changes by industries using or selling antibiotics, as well as time spend on research to establish causal links between antibiotic use and emergence of untreatable bacterial diseases. Today, there are two federal bills (S.742 and H.R. 2562) aimed at phasing out non-therapeutic antibiotics in US food animal production. These bills are endorsed by public health and medical organizations including the American Nurses Association (ANA), the American Academy of Pediatrics (AAP), and the American Public Health Association (APHA).
Humans[edit | edit source]
One study on respiratory tract infections found "physicians were more likely to prescribe antibiotics to patients who they believed expected them, although they correctly identified only about 1 in 4 of those patients".[7] Multifactorial interventions aimed at both physicians and patients can reduce inappropriate prescribing of antibiotics. [8] Delaying antibiotics for 48 hours while observing for spontaneous resolution of respiratory tract infections may reduce antibiotic usage; however, this strategy may reduce patient satisfaction.[9]
Excessive use of prophylactic antibiotics in travelers may also be classified as misuse.
Antibiotic resistance[edit | edit source]

Use or misuse of antibiotics may result in the development of antibiotic resistance by the infecting organisms, similar to the development of pesticide resistance in insects. Evolutionary theory of genetic selection requires that as close as possible to 100% of the infecting organisms be killed off to avoid selection of resistance; if a small subset of the population survives the treatment and is allowed to multiply, the average susceptibility of this new population to the compound will be much less than that of the original population, since they have descended from those few organisms which survived the original treatment. This survival often results from an inheritable resistance to the compound which was infrequent in the original population but is now much more frequent in the descendants thus selected entirely from those originally infrequent resistant organisms.

Antibiotic resistance has become a serious problem in both the developed and underdeveloped nations. By 1984 half of the people with active tuberculosis in the United States had a strain that resisted at least one antibiotic. In certain settings, such as hospitals and some child-care locations, the rate of antibiotic resistance is so high that the normal, low cost antibiotics are virtually useless for treatment of frequently seen infections. This leads to more frequent use of newer and more expensive compounds, which in turn leads inexorably to the rise of resistance to those drugs, and a race to discover new and different antibiotics ensues, just to keep us from losing ground in the battle against infection. The fear is that we will eventually fail to keep up in this race, and the time when people did not fear life-threatening bacterial infections will be just a memory of a golden era.
Another example of selection is Staphylococcus aureus, which could be treated successfully with penicillin in the 1940s and 1950s. At present, nearly all strains are resistant to penicillin, and many are resistant to nafcillin, leaving only a narrow selection of drugs such as vancomycin useful for treatment. The situation is worsened by the fact that genes coding for antibiotic resistance can be transferred between bacteria via plasmids, making it possible for bacteria never exposed to an antibiotic to acquire resistance from those which have. The problem of antibiotic resistance is worsened when antibiotics are used to treat disorders in which they have no efficacy, such as the common cold or other viral complaints, and when they are used widely as prophylaxis rather than treatment (as in, for example, animal feeds), because this exposes more bacteria to selection for resistance.
Beyond antibiotics[edit | edit source]
The comparative ease of identifying compounds which safely cured bacterial infections was more difficult to duplicate in treatments of fungal and viral infections. Antibiotic research led to great strides in the knowledge of biochemistry, establishing large differences between the cellular and molecular physiology of the bacterial cell and that of the mammalian cell. This explained the observation that many compounds that are toxic to bacteria are non-toxic to human cells. In contrast, the basic biochemistries of the fungal cell and the mammalian cell are much more similar. This restricts the development and use of therapeutic compounds that attack a fungal cell, while not harming mammalian cells. Similar problems exist in antibiotic treatments of viral diseases. Human viral metabolic biochemistry is very closely similar to human biochemistry, and the possible targets of antiviral compounds are restricted to very few components unique to a mammalian virus.
Research into bacteriophages for use as antibiotics is presently ongoing. Several types of bacteriophage appear to exist that are specific for each bacterial taxonomic group or species. Research into bacteriophages for medicinal use is just beginning, but has led to advances in microscopic imaging.[10] While bacteriophages provide a possible solution to the problem of antibiotic resistance, there is no clinical evidence yet that they can be deployed as therapeutic agents to cure disease.
Phage therapy has been used in the past on humans in the US and Europe during the 1920s and 1930s, but these treatments had mixed results. With the discovery of penicillin in the 1940s, Europe and the US changed therapeutic strategies to using antibiotics. However, in the former Soviet Union phage therapies continued to be studied. In the Republic of Georgia, the Eliava Institute of Bacteriophage, Microbiology & Virology continues to research the use of phage therapy. Various companies and foundations in North America and Europe are currently researching phage therapies.
References[edit | edit source]
- ↑ How Products Are Made: Antibiotics
- ↑ Robert Berkow (ed.) The Merck Manual of Medical Information - Home Edition. Pocket (September 1999), ISBN 0-671-02727-1.
- ↑ University of Michigan Health System: Antibiotic-Associated Diarrhea, November 26, 2006
- ↑ Pirotta MV, Garland SM (2006). "Genital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibiotics". J Clin Microbiol. 44: 3213–3217. PMID 16954250.
- ↑ Planned Parenthood: Does taking antibiotics make the pill less effective?, July 15, 2004
- ↑ Mellon, M et al. (2001) Hogging It!: Estimates of Antimicrobial Abuse in Livestock, 1st ed. Cambridge, MA: Union of Concerned Scientists.
- ↑ Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA (2007). "Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction". Annals of emergency medicine. 50 (3): 213–20. doi:10.1016/j.annemergmed.2007.03.026. PMID 17467120.
- ↑ Metlay JP, Camargo CA, MacKenzie T; et al. (2007). "Cluster-randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments". Annals of emergency medicine. 50 (3): 221–30. doi:10.1016/j.annemergmed.2007.03.022. PMID 17509729.
- ↑ Spurling G, Del Mar C, Dooley L, Foxlee R (2007). "Delayed antibiotics for respiratory infections". Cochrane database of systematic reviews (Online) (3): CD004417. doi:10.1002/14651858.CD004417.pub3. PMID 17636757.
- ↑ Purdue University "Biologists build better software, beat path to viral knowledge", see Imaging of Epsilon 15, a virus that infects the bacterium Salmonella News report
See also[edit | edit source]
External links[edit | edit source]
- Antibiotic News from Genome News Network (GNN)
- Are Antibiotics Killing Us? -Discover Magazine
- JAAPA: New antibiotics useful in primary care
- A new method for controlling bacterial activity without antibiotics - Research conducted at the Hebrew University
- BURDEN of Resistance and Disease in European Nations
Resources[edit | edit source]
Template:Major Drug Groups
Template:Throat preparations
af:Antibiotikum
ar:مضاد حيوي
bn:অ্যান্টিবায়োটিক
bs:Antibiotik
bg:Антибиотик
ca:Antibiòtic
cs:Antibiotikum
da:Antibiotikum
de:Antibiotika
el:Αντιβιoτικό
eo:Antibiotiko
fa:آنتیبیوتیک
fo:Antibiotika
gl:Antibiótico
ko:항생제
hr:Antibiotik
io:Antibiotiko
id:Antibiotik
it:Antibiotico
he:אנטיביוטיקה
lt:Antibiotikas
hu:Antibiotikum
nl:Antibioticum
no:Antibiotika
nn:Antibiotikum
qu:Unquy muhu wañuchiq
simple:Antibiotic
sk:Antibiotikum
sr:Антибиотик
fi:Antibiootti
sv:Antibiotikum
th:ยาปฏิชีวนะ
uk:Антибіотик
ur:ضد حیوی
yi:אנטיביאטיק
 KSF
KSF