Artificial pancreas
 From Wikidoc - Reading time: 14 min
From Wikidoc - Reading time: 14 min
The artificial pancreas is a technology in development to help diabetic persons automatically control their blood glucose level by providing the substitute endocrine functionality of a healthy pancreas.
There are several important exocrine (digestive) and endocrine (hormonal) functions of the pancreas, but it is the lack of insulin production which is the motivation to develop a substitute. While the current state of insulin replacement therapy is appreciated for its life-saving capability, the task of manually managing the blood sugar level with insulin alone is arduous and inadequate.
The goal of the artificial pancreas is twofold:
- to improve insulin replacement therapy until glycemic control is practically normal as evident by the avoidance of the complications of hyperglycemia, and
- to ease the burden of therapy for the insulin-dependent.
Different approaches under consideration include:
- the medical equipment approach -- using an insulin pump under closed loop control using real-time data from a continuous blood glucose sensor. This is emerging technology and will be the larger subject of this article.
- the bioengineering approach -- the development of a bio-artificial pancreas consisting of a biocompatible sheet of encapsulated beta cells. When surgically implanted, the islet sheet will behave as the endocrine pancreas and will be viable for years.
- the gene therapy approach -- the therapeutic infection of a diabetic person by a genetically engineered virus which causes a DNA change of intestinal cells to become insulin-producing cells.
Background in endocrine physiology[edit | edit source]
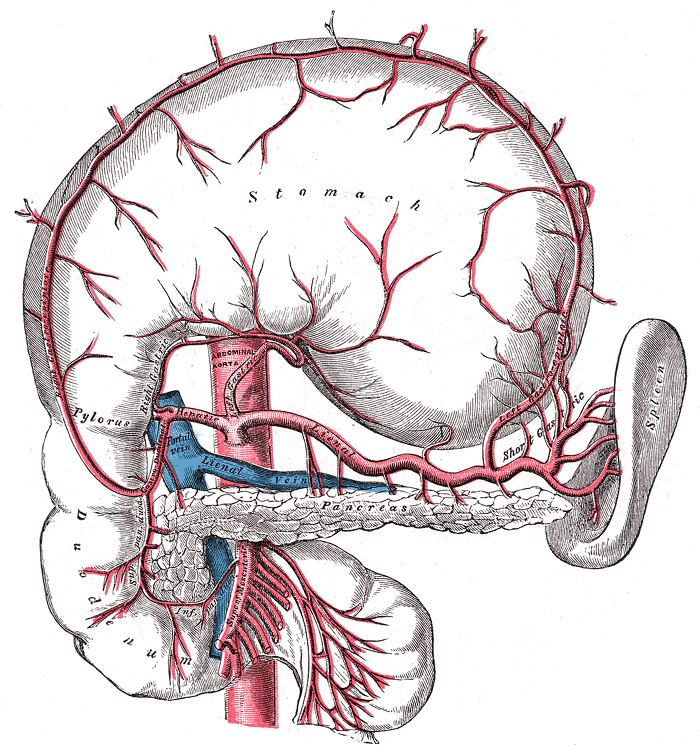
The pancreas produces three hormones that are important to glycemic control:
- insulin, which lowers blood glucose;
- amylin, which slows digestion and slows the rate of glucose entering the bloodstream, and temporarily suppresses release of glucagon;
- and glucagon, which raises the blood glucose.
Upon digestion of carbohydrates, glucose levels in the blood will begin to rise. As the blood and glucose flow into the pancreas, insulin and amylin are cosecreted by the pancreatic beta cells directly into the bloodstream in response to elevated blood glucose levels. Insulin causes blood glucose to be removed from the bloodstream and stored in the liver and muscle cells. Notice as the blood sugar goes higher, additional insulin will bring the blood sugar back down in a classic negative feedback loop. As insulin is released from the beta cells, amylin is also released into the bloodstream. Amylin slows gastric emptying, and also inhibits the release of glucagon from the pancreatic alpha cells. The effect of amylin is to spread out the blood glucose peak after eating, reducing the quantity of insulin needed. As the blood sugar level comes back toward normal, the beta cells will stop spurting insulin and amylin. As the glucose level approaches a low mark, the pancreatic alpha cells will release glucagon directly into the bloodstream. Glucagon causes the liver to release stored glucose back into the bloodstream. Notice that increased glucagon will increase blood glucose levels in a positive feedback loop. Together, the three endocrine hormones work as a system to control the blood glucose level between high and low boundaries.
When the beta cell produces insulin from proinsulin, a connecting peptide (or C-peptide) is also manufactured and released into the bloodstream. Absence of C-peptide in the blood indicates that insulin has not been released from the pancreas, and this fact confirms the diagnosis of diabetes type 1. C-peptide was believed to be only a by-product of natural insulin production, however recent studies suggest that C-peptide exerts beneficial therapeutic effects on diabetic nociceptive neuropathy.[1]
Ideally, to replicate the natural function of the pancreas as closely as possible, an artificial pancreas might someday replace all of the beneficial endocrine functions lost, including the delivery of insulin, amylin, glucagon, and C-peptide.
Background in insulin therapy[edit | edit source]
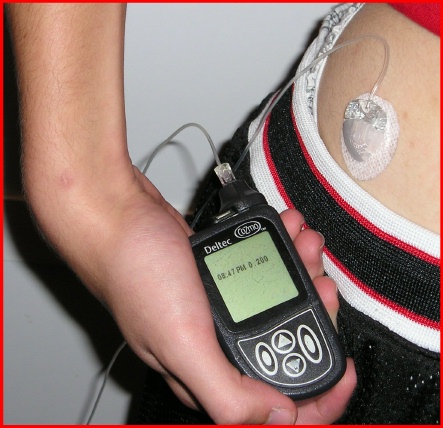
In insulin-dependent persons, blood glucose levels have been roughly controlled using insulin alone. The number of grams of carbohydrate is estimated by measuring foods, and is then used to determine the amount of insulin necessary to cover the meal. A simple open-loop model is used: an insulin to carbohydrate ratio (adjusted based on past success) is multiplied by the grams of carbohydrates to calculate the units of insulin needed. That quantity of insulin is then adjusted based on a pre-meal blood glucose measurement (insulin added for a high blood sugar and insulin removed for a low blood sugar). Insulin is injected or infused under the skin, and enters the bloodstream in approximately 15 minutes. After the insulin has acted in the bloodstream, the blood glucose level can be tested again and then adjusted with injection of more insulin, or eating more carbohydrates, until balance is restored.
There are notable differences with insulin replacement compared to the function of pancreatic insulin delivery:
- the insulin dose is predicted based on measured food (where accuracy of measured carbohydrate is difficult) whereas pancreatic insulin is released in proportional response to actual blood glucose levels;
- pancreatic insulin is released into to the portal vein, where it flows almost directly to the liver, which is the major organ for storing glycogen (50% of insulin produced is used by the liver);
- pancreatic insulin is pulsatile which helps maintain the insulin sensitivity of hepatic tissues;
- injected insulin is delivered subcutaneously (under the skin) but not directly to the bloodstream, so there is a delay before injected insulin begins to reduce blood glucose (although this can be compensated by injecting insulin 15 minutes before eating);
- replacement insulin therapy does not include amylin (although Symlin is now available for use), which can reduce the insulin need by 50%;
- replacement insulin is dosed as a best compromise between aggressive use for lowering the blood sugar when eating but also conservative use to avoid a post-prandial low blood sugar due to excess insulin, whereas pancreatic function releases insulin aggressively and later includes automatic release of glucagon at the end of an insulin cycle to manage the blood sugar level and avoid hypoglycemia.
An insulin pump to infuse a rapid-acting insulin is the first step in simulating the function of the pancreas. The pump can accurately deliver small increments of insulin compared to an injection, and its electronic controls permit shaping a bolus over time to match the insulin profile required for a given situation. However, the pump is still controlled manually by the pump user to bolus on command based on a snap shot of the recent blood glucose level and an estimate of the grams of carbohydrate consumed. This predictive approach is said to be open-loop. Once a bolus has been calculated and delivered, the pump continues to deliver its basal rate insulin in the manner that has been programmed into the pump controls based on the predicted insulin requirements of its user.
While insulin replacement is appreciated as a life saving therapy, its practical use in controlling blood glucose levels sufficiently to avoid the long term complications associated with hyperglycemia is not ideal.
Approaches to an Artificial Pancreas[edit | edit source]
Bioengineering approach[edit | edit source]

A biological approach to the artificial pancreas is to implant bioengineered tissue containing islet cells, which would secrete the amount on insulin, amylin, and glucagon needed in response to sensed glucose.
When islet cells have been transplanted via the Edmonton protocol, insulin production (and glycemic control) was restored, but at the expense of immunosuppression. Encapsulation of the islet cells in a protective coating has been developed to block the immune response to transplanted cells, which relieves the burden of immunosuppression and benefits the longevity of the transplant.[2]
One concept of the bio-artificial pancreas uses encapsulated islet cells to build an islet sheet which can be surgically implanted to function as an artificial pancreas.[3]
This islet sheet design consists of:
- an inner mesh of fibers to provide strength for the islet sheet;
- islet cells, encapsulated to avoid triggering a proliferating immune response, adhered to the mesh fibers;
- a semi-permeable protective layer around the sheet, to allow the diffusion of nutrients and secreted hormones;
- a protective coating, to prevent a foreign body response resulting in a fibrotic reaction which walls off the sheet and causes failure of the islet cells.
Islet sheet research is pressing forward with large animal studies at the present, with plans for human clinical trials within a few years.
Gene therapy approach[edit | edit source]
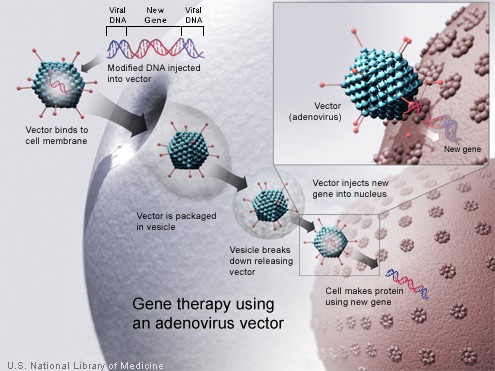
Technology for gene therapy is advancing rapidly such that there are multiple pathways possible to support endocrine function, with potential to practically cure diabetes.[4]
- Gene therapy can be used to manufacture insulin directly: an oral medication, consisting of viral vectors containing the insulin sequence, is digested and delivers its genes to the upper intestines. Those intestinal cells will then behave like any viral infected cell, and will reproduce the insulin protein. The virus can be controlled to infect only the cells which respond to the presence of glucose, such that insulin is produced only in the presence of high glucose levels. Due to the limited numbers of vectors delivered, very few intestinal cells would actually be impacted and would die off naturally in a few days. Therefore by varying the amount of oral medication used, the amount of insulin created by gene therapy can be increased or decreased as needed. As the insulin producing intestinal cells die off, they are boosted by additional oral medications.[5]
- Gene therapy might eventually be used to cure the cause of beta cell destruction, thereby curing the new diabetes patient before the beta cell destruction is complete and irreversible.[6]
- Gene therapy can be used to turn duodenum cells and duodenum adult stem cells into beta cells which produce insulin and amylin naturally. By delivering beta cell DNA to the intestine cells in the duodenum, a few intestine cells will turn into beta cells, and subsequently adult stem cells will develop into beta cells. This makes the supply of beta cells in the duodenum self replenishing, and the beta cells will produce insulin in proportional response to carbohydrates consumed.[7]
Medical equipment approach[edit | edit source]
Development of Continuous Blood Glucose Monitoring[edit | edit source]
Technology for continuous blood glucose monitoring supports the mission of the artificial pancreas by:
- automatically providing a blood glucose reading every few minutes without finger sticks from the user,
- monitoring trends pertaining to rising and falling blood sugars, which is helpful in the prediction of blood glucose levels in the immediate future,
- comparing blood sugar levels and predictions against a high blood sugar threshold, and then prompting the user that a correction bolus from an insulin pump is needed immediately,
- comparing blood sugar levels and predictions against a low blood sugar threshold, and then prompting the user to reduce the basal insulin from the pump or to eat something.
These capabilities suggest that a stream of real-time data can be used to "close the loop" and control the insulin pump directly.
Some issues with the present performance of continuous sensing technology suggest that additional study is needed for application to the artificial pancreas:
- continuous sensors require calibration a few times a day, by performing a manual blood glucose test with a finger stick, and then entering the blood glucose data into the continuous system for a sensor correction,
- continuous sensors are measuring interstitial glucose, so there is a time delay between the sensor data and the true blood glucose,
- automatic control removes the intellect of the user, which can be an additional safeguard when the data is subject to error and must be verified before taking action.
As the state of the art for blood glucose monitoring continues to advance, so does the promise of the artificial pancreas.
Feedback of real-time blood glucose data to an insulin pump for basal control[edit | edit source]
The first step in controlling an insulin pump based on continuous blood glucose data is to automatically control the basal rate of the insulin pump. When a bolus has not recently been performed, the pump can manage the blood glucose level by adjusting the basal rate as needed:
- when the blood sugar is increasing, a small correction bolus can be automatically delivered and a higher basal rate can be set;
- when the blood sugar is decreasing, the basal rate can be halted to deny the quantity of insulin needed to bring the blood glucose level back up until the basal rate can be continued at a new lower rate;
- and with adaptive filtering techniques, the pump can "learn" the unique basal rates for the person as a function of the time of day.
When controlling the basal rate alone, the closed loop can still correct a meal bolus error that was too large or small for the food consumed by:
- recognizing an imbalance between the bolus "insulin on board" and the level of blood glucose,
- automatically bolusing to correct a shortage of insulin,
- automatically reducing or interrupting the basal rate to correct an abundance of insulin,
- and using adaptive filtering techniques to "learn" the carbohydrate to insulin ratios for each meal bolus.
First clinical tests: implantable insulin pumps and continuous glucose sensors[edit | edit source]
In France, a human clinical trial of an artificial pancreas is underway. The system is fully automated by combining Medtronic MiniMed's long-term glucose sensor and its implantable insulin pump.[8] A summary of the project shows promise as well as some present limitations:
- The implantable sensor is inserted into a neck vein leading to the heart.
- The sensor is connected, via an electrical-type wire under the skin, to the implantable insulin pump: as blood sugar levels fluctuate, a signal tells the pump how much insulin to deliver.
- The sensor accurately measured glucose in 95% of cases when compared with values obtained by fingersticks.
- The blood glucose levels were maintained in the normal range more than 50% of the time in the patients using the pump connected to the sensor.
- Events of hypoglycemia dropped to less than 5%.
- While implantable insulin pumps work for an average of eight years before they have to be changed, the sensors stop working after an average of nine months,
- The mathematical programs that calculate just how much insulin should be delivered at different parts of the day also needs to be refined.
Insulin and amylin combination[edit | edit source]
When pramlintide (brand name Symlin or synthetic amylin) is used in combination with insulin, the benefits for post-prandial glycemic control are substantial.[9]
Pramlintide is a relatively new treatment for diabetes. The treatment involves:
- a separate injection of pramlintide before a meal,
- a reduction in insulin bolus by 50% for that meal.[10]
Pramlintide can be infused using an insulin pump. At the present time, the mixing of pramlintide and insulin in the same cartridge is not an approved practice, so two infusion pumps are used simultaneously. Since insulin and amylin are co-secreted by the pancreatic beta cells in response to raising blood glucose levels, using pramlintide and insulin together more closely duplicates the function of the pancreas.
Symlin has potential to support the artificial pancreas project because:
- Insulin and pramlintide may in the future be automatically infused together
- at a mixture from a single automatic insulin pump, or
- two infusion pumps could be used automatically with the insulin pump acting as master and the simlin pump acting as slave, or
- a dual system in one pump machine (two cartridges, a dual infusion set tube, and two subcutaneous insertions);
- because it improves post prandial glycemic excursions relative to insulin alone, this supports the possible use of an automatic bolus with less impact due to the delay of the insulin bolus;
- and because it simply duplicates the natural pancreas function, the full benefits of which are not fully understood.
Feedback of real-time blood glucose data to an insulin pump for bolus control[edit | edit source]
The ability of the electronic controls of the infusion pump, particularily in the bolus shaping capability, suggests that the control algorithm may replicate the function of the healthy pancreas in a more copycat fashion. At present, the insulin bolus is a predictive dose based on what is about to be eaten, and then infused completely. Even with the benefit of the closed-loop control of the basal insulin, the standard bolus is still a "guess and then fix it later" approach. Compare to the pancreatic physiology, where insulin and amylin are released from the beta cells in pulses almost directly to the liver in response to the immediate blood glucose level. The natural release from the beta cells is a closed loop response to sensed glucose, and the shape of the insulin delivery is adaptable and appropriate to the food eaten and the body's present metabolic capability.
As technology for continuous blood glucose monitoring improves, the integrated components will support a typical application of control theory by employing the proportional, integral, and derivative control algorithm.[11] This will make it feasible to infuse an adaptive bolus that changes its shape and integral dose based on the measured performance of the bolus in progress, depending on:
- the rate of glucose increase (i.e the derivative function would deliver more insulin for a rapid increase in blood sugar);
- the peak of the glucose curve (i.e. the proportional function would deliver more insulin for a higher peak in the blood sugar); and
- the duration of elevated glucose (i.e. the integral function would deliver more insulin for a long duration of high blood sugar).
The adaptive bolus could start with an assumption of a typical proportions and a bolus shape like the combination bolus. This could include:
- a prebolus of pramlintide (optional perhaps, but resolves issue with insulin timing)
- initiation of a combination bolus with the initial spike sized in proportion to the present blood glucose level and trends in the change of blood glucose level,
- modification to the square wave portion of the bolus, increasing or extending if blood sugar is increasing, and decreasing or limiting in duration when blood sugar is decreasing.
The benefits of an automatic bolus delivery might include:
- increased accuracy in the total insulin delivered relative to what was needed,
- freedom to the user of the artificial pancreas,
- elimination of glycemic excursions due to user error (such as forgetting to bolus in conventional pump therapy),
- adaptability to changes in digestion of carbohydrates based on food choices,
- adaptability to variable metabolic needs due to stress, illness, or exercise.
Glucagon combination[edit | edit source]
The purpose of glucagon is to raise blood sugar, primarily by promoting release of stored glucose in the liver. Human glucagon has been synthesized by recombinant DNA technology and is available in a dry powder form in the glucagon rescue kit. This is useful for rescue of unconscious diabetics from a severe state of hypoglycemia.[12]
In healthy pancreatic function, glucagon production is initially suppressed by beta cell production of insulin and amylin when blood sugar is high, and then is later produced by low or falling blood sugar. The natural pancreatic function uses glucagon at the end of an insulin cycle to release glucose from the liver, with two advantages:
- to prevent low blood sugar, and
- to speed the overall insulin action by cancelling the insulin tail.[13]
If an artificial pancreas was to simulate the natural endocrine pancreas to the maximum extent, then insulin and amylin would be used at the beginning of an insulin cycle and glucagon would be used at the end of the insulin cycle. While the copycat function of using glucagon seems desirable, the trade-off in cost and complexity relative to a gain, if any, beyond an artificial pancreas without glucagon is not known.
Initiatives around the globe[edit | edit source]
In the United States in 2006, the Juvenile Diabetes Research Foundation (JDRF) launched a multi-year initiative to help accelerate the availability of an artificial pancreas to people with diabetes.[14] The overall goal of the Artificial Pancreas Project is to accelerate the development, regulatory approval, and acceptance of continuous glucose monitoring and artificial pancreas technology in the shortest possible timeframe. The long term goal is for broad patient access and a thriving competitive market for these devices and products.
JDRF's role in quickening the development and availability of the Artificial Pancreas consists of funding research in order to look over the outcomes of patients using the Artificial Pancreas, keeping close contact with the Food and Drug Administration so that the standards of the patient are met, advocating for health care coverage of technologies such as the Artificial Pancreas and working to ensure clinical acceptance of technologies such as the Artificial Pancreas.
References[edit | edit source]
- ↑ http://diabetes.diabetesjournals.org/cgi/content/abstract/55/12/3581
- ↑ http://www.isletmedical.com/pages/define_methods.htm
- ↑ http://www.isletmedical.com/pages/company_research.htm
- ↑ http://www.niddk.nih.gov/fund/reports/gene_therapy_summ.htm
- ↑ http://www.liebertonline.com/doi/pdf/10.1089/dia.2005.7.549?cookieSet=1
- ↑ http://www.hopkinsbayview.org/healthcarenews06/060605diabetes.html
- ↑ http://www.engeneinc.com/
- ↑ http://www.medicinenet.com/script/main/art.asp?articlekey=52386
- ↑ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?itool=abstractplus&db=pubmed&cmd=Retrieve&dopt=abstractplus&list_uids=15498087
- ↑ http://www.symlin.com
- ↑ http://www.journalofdst.org/pdf/REVIEW/VOL-1-1-REV1-KLONOFF.pdf
- ↑ http://pi.lilly.com/glucagon-patient.pdf
- ↑ http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/glucagon.html
- ↑ http://www.jdrf.org/artificialpancreas
 KSF
KSF