Aspirin (delayed release tablet)
 From Wikidoc - Reading time: 10 min
From Wikidoc - Reading time: 10 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Aspirin (delayed release tablet) is an analgesic that is FDA approved for the treatment of minor aches and pains. Common adverse reactions include dyspepsia, hypoprothrombinemia, thrombocytopenia, hypersensitivity, cholestatic hepatitis, and tinnitus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Temporarily relieves minor aches and pains.
- For other uses, see your doctor, but do not use for more than 10 days without consulting your doctor because serious side effects may occur.
Dosage
- Drink a full glass of water with each dose.
- Adults and children 12 years and over: take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours unless directed by a doctor.
- Children under 12 years: consult a doctor.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aspirin (delayed release tablet) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aspirin (delayed release tablet) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Aspirin (delayed release tablet) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aspirin (delayed release tablet) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aspirin (delayed release tablet) in pediatric patients.
Contraindications
There is limited information regarding Aspirin (delayed release tablet) Contraindications in the drug label.
Warnings
- Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
- Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- Hives
- Facial swelling
- Shock
- Asthma (wheezing)
- Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- Are age 60 or older
- Have had stomach ulcers or bleeding problems
- Take a blood thinning (anticoagulant) drug
- Take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- Have 3 or more alcoholic drinks every day while using this product
- Take more or for a longer time than directed.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Aspirin (delayed release tablet) in the drug label.
Postmarketing Experience
Dyspepsia, hypoprothrombinemia, thrombocytopenia, hypersensitivity, cholestatic hepatitis, tinnitus.
Drug Interactions
There is limited information regarding Aspirin (delayed release tablet) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aspirin (delayed release tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aspirin (delayed release tablet) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Aspirin (delayed release tablet) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Aspirin (delayed release tablet) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Aspirin (delayed release tablet) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Aspirin (delayed release tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aspirin (delayed release tablet) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Aspirin (delayed release tablet) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Aspirin (delayed release tablet) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aspirin (delayed release tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aspirin (delayed release tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
There is limited information regarding Monitoring of Aspirin (delayed release tablet) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Aspirin (delayed release tablet) in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Aspirin (delayed release tablet) in the drug label.
Pharmacology
Mechanism of Action
There is limited information regarding Aspirin (delayed release tablet) Mechanism of Action in the drug label.
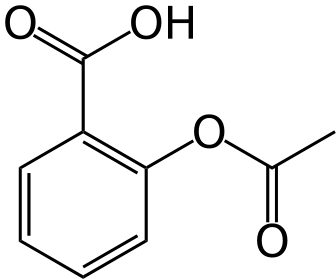
Structure
There is limited information regarding Aspirin (delayed release tablet) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Aspirin (delayed release tablet) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Aspirin (delayed release tablet) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Aspirin (delayed release tablet) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Aspirin (delayed release tablet) in the drug label.
How Supplied
There is limited information regarding Aspirin (delayed release tablet) How Supplied in the drug label.
Storage
There is limited information regarding Aspirin (delayed release tablet) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Aspirin (delayed release tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Aspirin (delayed release tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Aspirin (delayed release tablet) in the drug label.
Precautions with Alcohol
- Alcohol-Aspirin (delayed release tablet) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ASPIRIN DELAYED RELEASE®[3]
Look-Alike Drug Names
There is limited information regarding Aspirin (delayed release tablet) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 "Zorprin, Bayer Buffered Aspirin (aspirin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 3 April 2014.
- ↑ 2.0 2.1 2.2 Brayfield, A, ed. (14 January 2014). "Aspirin". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 3 April 2014.
- ↑ "ASPIRIN DELAYED RELEASE- aspirin tablet, delayed release".
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Butalbital_Aspirin_and_Caffeine_NDC_01431785.jpg |Drug Name=Butalbital, Aspirin and Caffeine |Pill Ingred=BUTALBITAL[BUTALBITAL];ASPIRIN[ASPIRIN];CAFFEINE[CAFFEINE]|+sep=; |Pill Imprint=Westward;785 |Pill Dosage=325 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=West-ward Pharmaceutical Corp |NDC=01431785
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aspirin_NDC_05363086.jpg |Drug Name=Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=AP;121 |Pill Dosage=81 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Rugby Laboratories Inc. |NDC=05363086
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Micro-Coated_Aspirin_NDC_05363305.jpg |Drug Name=Micro-Coated Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=TCL;011 |Pill Dosage=325 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=Rugby Laboratories, Inc |NDC=05363305
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Enteric_Coated_Aspirin_NDC_05363313.jpg |Drug Name=Enteric Coated Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=T |Pill Dosage=325 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Rugby Laboratories, Inc |NDC=05363313
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Butalbital_Aspirin_Caffeine_and_Codeine_Phosphate_NDC_05913546.jpg |Drug Name=Butalbital, Aspirin, Caffeine and Codeine Phosphate |Pill Ingred=CODEINE PHOSPHATE[CODEINE ANHYDROUS];BUTALBITAL[BUTALBITAL];CAFFEINE[CAFFEINE];ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=WATSON;3546 |Pill Dosage=325 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=Watson Laboratories, Inc. |NDC=05913546
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aspirin_NDC_06030024.jpg |Drug Name=Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=AZ013 |Pill Dosage=81 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceutical, Inc. |NDC=06030024
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Enteric_Coated_Aspirin_NDC_06030169.jpg |Drug Name=Enteric Coated Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=T;A |Pill Dosage=325 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06030169
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Butalbital_Aspirin_and_Caffeine_Tablets_NDC_06032548.jpg |Drug Name=Butalbital, Aspirin and Caffeine Tablets |Pill Ingred=ASPIRIN[ASPIRIN];BUTALBITAL[BUTALBITAL];CAFFEINE[CAFFEINE]|+sep=; |Pill Imprint=West;ward;785 |Pill Dosage=325 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest |NDC=06032548
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=aspirin_regular_strength_NDC_09042009.jpg |Drug Name=aspirin regular strength |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=Aspirin;A1 |Pill Dosage=325 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09042009
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Regular_Strength_Aspirin_EC_NDC_09042013.jpg |Drug Name=Regular Strength Aspirin EC |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=T |Pill Dosage=325 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals Inc |NDC=09042013
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Tri-Buffered_Aspirin_NDC_09042015.jpg |Drug Name=Tri-Buffered Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=44;183 |Pill Dosage=325 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09042015
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aspirin_NDC_09044040.jpg |Drug Name=aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=L467 |Pill Dosage=81 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09044040
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aspirin_NDC_09046288.jpg |Drug Name=aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=TCL;334;UPPER;LOWER;PLAIN |Pill Dosage=81 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09046288
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aspir_Low_NDC_09047704.jpg |Drug Name=Aspir Low |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=N |Pill Dosage=81 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09047704
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Low_Strength_Chewable_Aspirin_NDC_578960911.jpg |Drug Name=Low Strength Chewable Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=AZ;013 |Pill Dosage=81 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Geri-Care Pharmaceutical Corp |NDC=578960911
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Regular_Strength_Enteric_coated_aspirin_NDC_578960921.jpg |Drug Name=Regular Strength Enteric coated aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=44;227 |Pill Dosage=325 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Geri-Care Pharmaceutical Corp |NDC=578960921
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Regular_Strength_Aspirin_EC_NDC_675440468.jpg |Drug Name=Regular Strength Aspirin EC |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=T |Pill Dosage=325 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Aphena Pharma Solutions - Tennessee, LLC |NDC=675440468
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aspirin_EC_NDC_01821061.jpg |Drug Name=Aspirin EC |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=N |Pill Dosage=81 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Goldline Laboratories, Inc. |NDC=01821061
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Butalbital_Aspirin_and_Caffeine_NDC_05913219.jpg |Drug Name=Butalbital, Aspirin, and Caffeine |Pill Ingred=BUTALBITAL[BUTALBITAL];ASPIRIN[ASPIRIN];CAFFEINE[CAFFEINE]|+sep=; |Pill Imprint=WATSON;3219 |Pill Dosage=325 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=Watson Laboratories, Inc. |NDC=05913219
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aggrenox_NDC_05970001.jpg |Drug Name=Aggrenox |Pill Ingred=aspirin[aspirin];dipyridamole[dipyridamole]|+sep=; |Pill Imprint=01A; |Pill Dosage=25 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=24 |Pill Scoring=1 |Pill Image= |Drug Author=Boehringer Ingelheim Pharmaceuticals, Inc. |NDC=05970001
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Regular_Strength_Aspirin_EC_NDC_09042011.jpg |Drug Name=Regular Strength Aspirin EC |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=T |Pill Dosage=325 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals Inc |NDC=09042011
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aspirin_NDC_508440157.jpg |Drug Name=Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=ASPIRIN;44157 |Pill Dosage=325 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=L.N.K. International, Inc. |NDC=508440157
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Aspirin_NDC_508440249.jpg |Drug Name=Aspirin |Pill Ingred=ASPIRIN[ASPIRIN]|+sep=; |Pill Imprint=ASPIRIN;44249 |Pill Dosage=325 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=L.N.K. International, Inc. |NDC=508440249
}}
{{#subobject:
|Page Name=Aspirin (delayed release tablet) |Pill Name=Ascomp_with_Codeine_NDC_519910074.jpg |Drug Name=Ascomp with Codeine |Pill Ingred=Butalbital[Butalbital];Aspirin[Aspirin];Caffeine[Caffeine];Codeine Phosphate[Codeine]|+sep=; |Pill Imprint=B074 |Pill Dosage=325 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=Breckenridge Pharmaceutical, Inc. |NDC=519910074
}}
 KSF
KSF