Bovine spongiform encephalopathy pathophysiology
 From Wikidoc - Reading time: 3 min
From Wikidoc - Reading time: 3 min
|
Bovine Spongiform Encephalopathy Microchapters |
|
Differentiating Bovine Spongiform Encephalopathy from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Bovine spongiform encephalopathy pathophysiology On the Web |
|
American Roentgen Ray Society Images of Bovine spongiform encephalopathy pathophysiology |
|
Bovine spongiform encephalopathy pathophysiology in the news |
|
Directions to Hospitals Treating Bovine spongiform encephalopathy |
|
Risk calculators and risk factors for Bovine spongiform encephalopathy pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] ; Associate Editor(s)-in-Chief: Adnan Ezici, M.D[2]
Overview[edit | edit source]
It is understood that Bovine spongiform encephalopathy (BSE) is caused by a misfolded prion protein. Misfolded prion proteins carry the disease between individuals and cause deterioration of the brain. BSE is a type of transmissible spongiform encephalopathy (TSE).
Pathophysiology[edit | edit source]
Physiology[edit | edit source]
The normal physiologic functions of prion protein (PrPC) can be understood as follows:[1]
- Protection against oxidative stress
- Neuroprotection
- Neuronal differentiation
- Neuronal excitability
- Myelin maintenance
- Regulation of circadian rhythm
- Iron homeostasis
- Regulation of signaling pathways (e.g., ERK1/2, PI3K-Akt, cAMP-PKA, etc.)
Pathogenesis[edit | edit source]
It is understood that Bovine spongiform encephalopathy (BSE) is caused by a misfolded prion protein. Misfolded prion proteins carry the disease between individuals and cause deterioration of the brain. BSE is a type of transmissible spongiform encephalopathy (TSE).[2] TSEs can arise in animals that carry an allele which causes normal prions to contort by themselves from an alpha-helical arrangement to a beta-pleated sheet, which is the disease-causing shape for the particular protein. Transmission can occur when healthy animals come in contact with tainted tissues from others with the disease. In the brain, these proteins cause native cellular prion protein to deform into the infectious state, which then goes on to deform further prion protein in an exponential cascade. This results in protein aggregates, which then form dense plaque fibers, leading to the microscopic appearance of "holes" in the brain, degeneration of physical and mental abilities, and ultimately death.
Different theories exist for the origin of prion proteins in cattle. Two leading theories suggest that it may have jumped species from the disease scrapie in sheep, or that it evolved from a spontaneous form of "mad-cow disease" which has been seen occasionally in cattle for many centuries.[3] The British Government enquiry took the view the cause was not scrapie as had originally been postulated, and was some event in the 1970s which was not possible to identify.[4]
Genetics[edit | edit source]
Genes that might be involved in the pathogenesis of bovine spongiform encephalopathy (BSE) include:[5]
- PRNP gene
- SPRN gene
Associated Conditions[edit | edit source]
Conditions associated with bovine spongiform encephalopathy include:
Gross Pathology[edit | edit source]
There is no characteristic finding of BSE on gross pathological examination.[6]
Microscopic Pathology[edit | edit source]
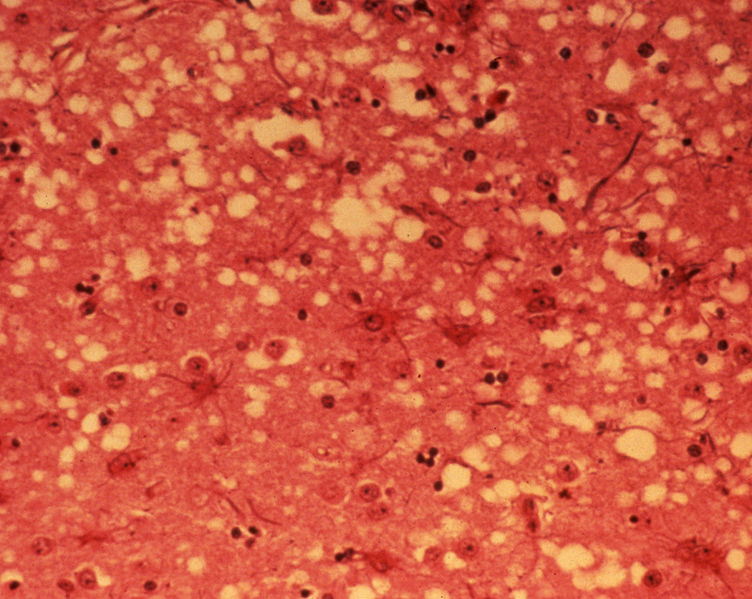
On microscopic histopathological analysis, microcavitation or vacuolization of the neuropil in grey matter nuclei of the brainstem, and intracytoplasmic vacuolization of the neural perikarya and axons of the brainstem nuclei (which gives the impression of "spongy brain") are characteristic findings of BSE, which usually involve the brainstem, bilateral and symmetrical.[6][7]
References[edit | edit source]
- ↑ Castle AR, Gill AC (2017). "Physiological Functions of the Cellular Prion Protein". Front Mol Biosci. 4: 19. doi:10.3389/fmolb.2017.00019. PMC 5382174. PMID 28428956.
- ↑ "An Overview of Bovine Spongiform Encephalopaphy" (PDF).
- ↑ New Scientist, 17 March 2007, p 11
- ↑ http://www.bseinquiry.gov.uk/report/volume1/execsum4.htm
- ↑ Murdoch BM, Murdoch GK (2015). "Genetics of Prion Disease in Cattle". Bioinform Biol Insights. 9 (Suppl 4): 1–10. doi:10.4137/BBI.S29678. PMC 4589088. PMID 26462233.
- ↑ 6.0 6.1 Davis AJ, Jenny AL, Miller LD (July 1991). "Diagnostic characteristics of bovine spongiform encephalopathy". J Vet Diagn Invest. 3 (3): 266–71. doi:10.1177/104063879100300318. PMID 1912001.
- ↑ Detwiler LA, Rubenstein R (2000). "Bovine spongiform encephalopathy: an overview". ASAIO J. 46 (6): S73–9. doi:10.1097/00002480-200011000-00041. PMID 11110298.
 KSF
KSF