Carbocation
 From Wikidoc - Reading time: 4 min
From Wikidoc - Reading time: 4 min
A carbocation (IPA pronunciation: Template:IPA) is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability (octet rule). Therefore carbocations are often reactive, seeking to fill the octet of valence electrons as well as regain a neutral charge. A typical carbocation has sp2 hybridization with a trigonal planar molecular geometry.
Definitions[edit | edit source]
A carbocation was previously often called a carbonium ion but questions arose on the exact meaning [1]. In present day chemistry a carbocation is any positively charged carbon atom. Two special types have been suggested: carbenium ions are trivalent and carbonium ions are pentavalent or hexavalent. University level textbooks only discuss carbocations as if they are carbenium ions [2], or discuss carbocations with a fleeting reference to the older phrase of carbonium ion [3] or carbenium and carbonium ions [4]. One textbook to this day clings on to the older name of carbonium ion for carbenium ion and reserves the phrase hypervalent carbenium ion for CH5+ [5].
History[edit | edit source]
The history of carbocations dates back to 1891 when G. Merling [6] reported that he added bromine to tropylidene (cycloheptatriene) and then heated the product to obtain a crystalline, water soluble material, C7H7Br. He did not suggest a structure for it; however Doering and Knox [7] convincingly showed that it was tropylium (cycloheptatrienylium) bromide. This ion is predicted to be aromatic by the Hückel Rule.
In 1902 Norris and Kehrman independently discovered that colorless triphenylmethanol gave deep yellow solutions in concentrated sulfuric acid. Triphenylmethyl chloride similarly formed orange complexes with aluminium and tin chlorides. Adolf von Baeyer recognized in 1902 the salt like character of the compounds formed.
- Ph3C-OH + H2SO4 Template:Unicode Ph3C+HSO4- + H2O (Ph stands for a phenyl substituent)
He dubbed the relationship between color and salt formation halochromy of which malachite green is a prime example.
Carbocations are reactive intermediates in many organic reactions. This idea, first proposed by Julius Stieglitz in 1899 (On the Constitution of the Salts of Imido-Ethers and other Carbimide Derivatives; Am. Chem. J. 21, 101; ISSN: 0096-4085) was further developed by Hans Meerwein in his 1922 study [8] of the Wagner-Meerwein rearrangement. Carbocations were also found to be involved in the SN1 reaction and E1 reaction and in rearrangement reactions such as the Whitmore 1,2 shift. The chemical establishment was reluctant to accept the notion of a carbocation and for a long time the Journal of the American Chemical Society refused articles that mentioned them.
The first NMR spectrum of a stable carbocation in solution was published by Doering et al. [9]. It was the heptamethylbenzenonium ion, made by treating hexamethylbenzene with methyl chloride and aluminium chloride. The stable 7-norbornadienyl cation was prepared by Story et al. [10] by reacting norbornadienyl chloride with silver tetrafluoroborate in sulfur dioxide at -80°C . The NMR spectrum established that it was nonclassically bridged (the first stable non-classical ion observed).
In 1962 Olah directly observed the tert-butyl carbocation by Nuclear magnetic resonance as a stable species on dissolving tert-butyl fluoride in magic acid. The NMR of norbornyl cation was first reported by Schleyer et al. [11] and it was shown to undergo proton scrambling over a barrier by Saunders et al. [12].
Properties[edit | edit source]
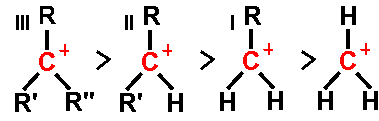
tertiary ( III ), secondary ( II ), and primary ( I ) alkyl carbocations
In organic chemistry, a carbocation is often the target of nucleophilic attack by nucleophiles like OH- ions or halogen ions.
Carbocations are classified as primary, secondary, or tertiary depending on the number of carbon atoms bonded to the ionized carbon. Primary carbocations have one or zero carbons attached to the ionized carbon, secondary carbocations have two carbons attached to the ionized carbon, and tertiary carbocations have three carbons attached to the ionized carbon.
Stability of the carbocation increases with the number of alkyl groups bonded to the charge-bearing carbon. Tertiary carbocations are more stable (and form more readily) than secondary carbocations; primary carbocations are highly unstable because, while ionized higher-order carbons are stabilized by Hyperconjugation, unsubstituted (primary) carbons are not. Therefore, reactions such as the SN1 reaction and the E1 elimination reaction normally do not occur if a primary carbocation would be formed. An exception to this occurs when there is a carbon-carbon double bond next to the ionized carbon. Such cations as allyl cation CH2=CH-CH2+ and benzyl cation C6H5-CH2+ are more stable than most other carbocations. Molecules which can form allyl or benzyl carbocations are especially reactive.
Carbocations undergo rearrangement reactions from less stable structures to equally stable or more stable ones with rate constants in excess of 1.0E9/sec. This fact complicates synthetic pathways to many compounds. For example, when 3-pentanol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a statistical mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce about 1/3 3-chloropentane and 2/3 2-chloropentane.
Some carbocations such as the norbornyl cation exhibit more or less symmetrical three centre bonding. Cations of this sort have been referred to as non-classical ions. The energy difference between "classical" carbocations and "non-classical" isomers is often very small, and there is generally little, if any activation energy involved in the transition between "classical" and "non-classical" structures. The "non-classical" form of the 2-butyl carbocation is essentially 2-butene with a proton directly above the centre of what would be the carbon-carbon double bond. "Non-classical" carbocations were once the subject of great controversy. One of George Olah's greatest contributions to chemistry was resolving this controversy [13].
References[edit | edit source]
- ↑ Gold Book definition Link
- ↑ Organic chemistry 5th Ed. John McMurry ISBN 0534376177
- ↑ Organic Chemistry, Fourth Edition Paula Yurkanis Bruice ISBN 0131407481
- ↑ Organic Chemistry Jonathan Clayden, Nick Geeves, Stuart Warren First Edition ISBN 0198503466
- ↑ Organic Chemistry by Marye Anne Fox and James K. Whitesell ISBN 076370413X
- ↑ Chem. Ber. 24, 3108 1891
- ↑ The Cycloheptatrienylium (Tropylium) Ion W. Von E.Doering and L. H. Knox J. Am. Chem. Soc.; 1954; 76(12) pp 3203 - 3206; doi:10.1021/ja01641a027
- ↑ H. Meerwein and K. van Emster, Berichte, 1922, 55, 2500.
- ↑ The 1,1,2,3,4,5,6-heptamethylbenzenonium ion W. von E. Doering and M. Saunders H. G. Boyton, H. W. Earhart, E. F. Wadley and W. R. Edwards G. Laber Tetrahedron Volume 4, Issues 1-2 , 1958, Pages 178-185 doi:10.1016/0040-4020(58)88016-3
- ↑ The 7-norbornadienyl carbonium ion Paul R. Story and Martin Saunders J. Am. Chem. Soc.; 1960; 82(23) pp 6199 - 6199; doi:10.1021/ja01508a058
- ↑ Stable Carbonium Ions. X.1 Direct Nuclear Magnetic Resonance Observation of the 2-Norbornyl Cation Paul von R. Schleyer, William E. Watts, Raymond C. Fort, Melvin B. Comisarow, and George A. Olah J. Am. Chem. Soc.; 1964; 86(24) pp 5679 - 5680; doi:10.1021/ja01078a056
- ↑ Stable Carbonium Ions. XI.1 The Rate of Hydride Shifts in the 2-Norbornyl Cation Martin Saunders, Paul von R. Schleyer, and George A. Olah J. Am. Chem. Soc.; 1964; 86(24) pp 5680 - 5681; doi:10.1021/ja01078a057
- ↑ http://nobelprize.org/chemistry/laureates/1994/olah-lecture.html
 KSF
KSF