Cardiac electrophysiology
 From Wikidoc - Reading time: 10 min
From Wikidoc - Reading time: 10 min
| Cardiac electrophysiology | |
 | |
|---|---|
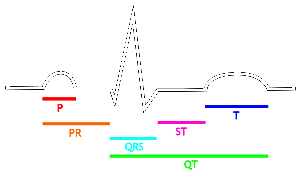
| Drawing of the EKG, with labels of intervals |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Assistant Editor(s)-in-Chief: Rim Halaby; Serge Korjian
Overview[edit | edit source]
- Cardiac electrophysiology (also referred to as clinical cardiac electrophysiology , or electrophysiology) is the science of the mechanisms, functions, and performance of the electrical activities of specific regions of the heart.
- The normal electrical conduction in the heart allows the impulse that is generated by the sinoatrial node (SA node) of the heart to be propagated to (and stimulate) the myocardium (Cardiac muscle). The myocardium contracts after stimulation. It is the ordered stimulation of the myocardium that allows efficient contraction of the heart, thereby allowing blood to be pumped throughout the body.
Cardiac Conduction System[edit | edit source]
- Proper cardiac function heavily depends on the ability of the cardiomyocytes to receive and propagate an electrical impulse allowing the heart to contract.
- These impulses, known as action potentials, originate and travel through the cardiac conduction system.
- A time-ordered propagation of the electrical impulse through the myocardium allows efficient contraction of all four chambers of the heart, starting with the atria pumping the blood toward the ventricles, followed by the ventricles which contribute to the pulmonary and systemic circulation.
The Components of the Cardiac Conduction System:[edit | edit source]
- The sinus (sinoatrial) node
- The internodal tracts
- The atrioventricular (AV) node
- The His/AV bundle
- The right and left bundle branches,
- The Purkinje fibers.
The Direction of Propagation of the Action Potential:[edit | edit source]
- The initial cardiac impulse, produced by pacemaker cells, originates in the sinoatrial (SA) node at the intersection of the right atrium and the superior vena cava.
- This action potential is the trigger of every cardiac cycle, initiating the atrial then ventricular contractions; it is henceforth responsible for the rhythmic beating of the heart.
- This action potential then propagates as a wave of depolarization through the internodal tracts initiating atrial contraction and then converging at the AV node.
- The convergence occurs because, in a normal heart, the AV node is the only electrical connection between the atria and the ventricles.
- The conduction of this potential is delayed at the AV node mainly due to the slower depolarization in these cells.
- This delay is represented as the PR interval of the ECG.
- The electrical impulse then moves to the ventricles by means of the AV or His bundle located in the superior portion of the interventricular septum.
- It then continues moving apically and propagating through both [[]]ventricles via the right and left bundle branches, and the Purkinje fibers.[1][2][3][4]
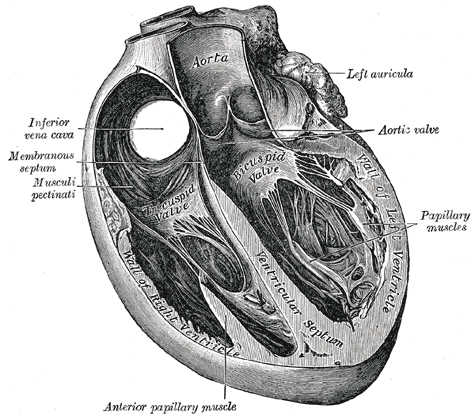
-
Section of the heart showing the ventricular septum.
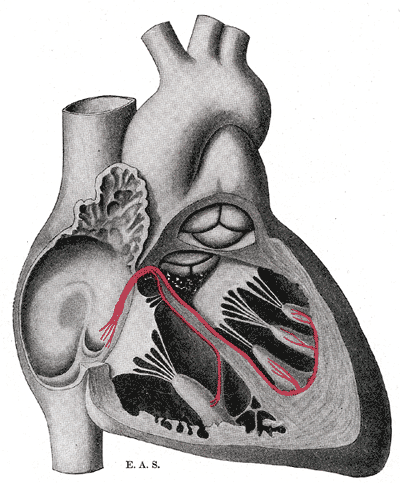
-
Schematic representation of the atrioventricular bundle of His.
Normal Cardiac Action Potential[edit | edit source]
- Similarly to skeletal muscle cells which are also striated muscles, the cardiomyocytes contract in response to a rapid alteration in the cell membrane’s potential.
- However, the cardiomyocytes differ from skeletal muscle cells by three important variations that are essential for cardiac function:
- 1) They can self-generate a change in cell membrane potential.
- 2) The action potential can be transmitted directly from cell to cell.
- 3) The action potentials have a significantly longer duration.
The Resting Membrane Potential[edit | edit source]
- All cells including cardiomyocytes have a resting membrane potential that is maintained assuming there is no electrical charge crossing the membrane from the intracellular towards the extracellular milieu or vice versa.
- This potential is estimated to be –80 to –90 mV.
- The most crucial ions that determine this resting potential are:
- Sodium (Na+)
- Calcium (Ca2+)
- Potassium (K+)
- Sodium (Na+) and calcium (Ca2+) are most present in the interstitial fluid, while potassium (K+) is more present in intracellularly.
- These ions are lipid insoluble which prevents them from crossing the lipid bilayer or the cell membrane.
- Alternatively, ions cross via specific protein structures in the cell membrane that may be either: ion channels, ion pumps, or ion exchangers.
- These transmembrane proteins are highly specific and allow only one type of ion to pass through which allows good maintenance of the membrane potential.
- Ion channels can be opened, inactivated or closed depending on complex factors that modulate their activity.
The Cardiac Action Potential[edit | edit source]
- The cardiac contraction action potential is divided into 5 phases.
Phase 0: Depolarization[edit | edit source]
- The initial rapid increase in the transmembrane potential from -80mV to approximately +30mV constitutes the Phase 0 or the depolarization phase.
- This depolarization results from a rapid increase in the membrane permeability to Na+ ions via opening of voltage-dependent fast Na+ channels allowing Na+ ions to move intracellularly according to their electrochemical gradient.
- Following the conduction of an action potential, a recovery phase is attained where a large number of Na+ channels are inactivated, preventing the conduction of a second action potential.
- When the membrane is fully repolarized, these channels are reactivated and allow the conduction of the following action potential.
Phase I: Initial Repolarization[edit | edit source]
- The phase I of the action potential, known as the initial rapid repolarization ensues, resulting from K+ and Cl- ion flux across the membrane.
- This forms the notch seen in the action potential following the depolarization.
Phase II: Plateau[edit | edit source]
- Phase II, almost exclusive to cardiomyocytes, represents a plateau in the membrane potential as an outcome of the equilibrium between Ca2+ influx and K+ outflow.
- The channels responsible for this Ca2+ influx are known as the L-type calcium channels, which are activated rapidly when the membrane potential reaches -50mV, but are slowly inactivated thereafter.
- Throughout this plateau phase, few Na+ channels also remain active.
- These are Na+/Ca2+ exchangers that allow 1 ion of calcium to move outside the cell for every 3 molecules of sodium moving inside the cell.
Phase III: Repolarization[edit | edit source]
- The third phase, also known as rapid repolarization, depicts the restoration of a resting membrane potential.
- It is initiated by inactivation of the L-type calcium channels and an increase in K+ outflow.
- This change in potassium across the membrane is related to 3 K+ currents:
- 1) Inwardly rectifying K+ current (IK1) à Produces the resting membrane potential
- 2) Transient outward K+ current (ITO) à Accounts for initial part of repolarization
- 3) Delayed outward K+ current (IK) à Responsible for final part of repolarization
- After repolarization has occurred, intracellular Na+ and extracellular K+ are rearranged via the Na+/K+ ATPase pump.
- The ATPase moves 3 sodium ions out for every 2 potassium ions moved intracellularly.
- Equilibrium of ions across the membrane is also achieved via the Na+/Ca2+ exchangers.
Phase IV: Diastolic depolarization[edit | edit source]
- The phase IV of the action potential is characterized by a diastolic depolarization that is both spontaneous and slow.
- This phase provides cardiac cells with features of automaticity.
- In a normal functioning heart, only the sinoatrial node is able to reach a threshold potential during phase IV making it the pacemaker of the heart.
- Nevertheless other cells including those in the AV node, the His bundle, and the Purkinje fibers are able to reach a threshold and fire automatically if they are not suppressed by the SA node, which is true in some disease entities.
The factors responsible for the initial diastolic depolarization in the SA node are:[edit | edit source]
- Inward Ca2+ current
- Delayed outward K+ current
- IF Currents – Inward sodium-potassium currents activated if membrane repolarizes below the If threshold
- T-type Ca2+ channel – Releases calcium from internal stores
The rate of impulse generation by the SA node is determined by 3 factors:[edit | edit source]
- 1) The slope of diastolic depolarization
- 2) The maximal diastolic potential
- 3) The threshold potential
- All these factors are controlled by the autonomic nervous system allowing for the modulation of the rate of SA node firing and subsequently the heart rate.[1][5][3][4]
Electrophysiology Studies and Therapeutic Modalities[edit | edit source]
Overview[edit | edit source]
- An electrophysiologic study is a term used to describe a number of invasive (intracardiac) and non-invasive recording of spontaneous electrical activity as well as of cardiac responses to programmed electrical stimulation.
- These studies are performed to assess arrhythmias, elucidate symptoms, evaluate abnormal electrocardiograms, assess risk of developing arrhythmias in the future, and design treatment.
- These procedures increasingly include therapeutic methods (typically radiofrequency ablation) in addition to diagnostic and prognostic procedures.
- Other therapeutic modalities employed in this field include antiarrhythmic drug therapy and implantation of pacemakers and implantable cardioverter-defibrillators.
- A specialist in cardiac electrophysiology is known as a cardiac electrophysiologist, or (more commonly) simply an electrophysiologist. Cardiac electrophysiology is considered a subspecialty of cardiology, and in most countries requires two or more years of fellowship training beyond a general cardiology fellowship. They are trained to perform interventional cardiac EP procedures as well as surgical device implantations.
Diagnostic Testing[edit | edit source]
- Ambulatory electrocardiographic monitoring (Holter recording and interpretation; loop recording and interpretation)
- Tilt table testing
- Signal-averaged electrocardiogram (SAECG) interpretation, also referred to as "late potentials" reading
- Electrophysiologic study (EPS)
- Pacing and recording electrodes are inserted either in the esophagus (intra-esophageal EPS) or, through blood vessels, directly into the heart chambers (intra-cardiac EPS) in order to measure electrical properties of the heart. In addition, intra-cardiac EPS electrically stimulates the heart and induces arrhythmias for diagnostic purposes ("programmed electrical stimulation").
Medical Treatment[edit | edit source]
Electrophysiologists play a role in:
- The initial administration and monitoring of the effect of drugs for treatment of heart rhythm disorders
- The management of severe or life threatening arrhythmias
- The management of arrythmias requiring multiple drugs use
Catheter Ablation[edit | edit source]
- Ablation therapy is the catheter based creation of lesions in the heart with radiofrequency energy, cryotherapy (destructive freezing), or ultrasound energy in order to cure or control arrhythmias (see radiofrequency ablation). Ablation is usually performed during the same procedure as the electrophysiology study which induces and confirms the diagnosis of the arrhythmia for which ablation therapy is sought.
Non-complex ablation[edit | edit source]
- It includes ablation for arrhythmias such as: AV nodal reentrant tachycardia, accessory pathway mediated tachycardia and atrial flutter.
- This procedure is usually performed using intracardiac catheters , fluoroscopy (a real-time X-ray camera), and electrical recordings from the inside of the heart.
Complex ablation[edit | edit source]
- It includes ablation for arrhythmias such as: multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia.
- In addition to the apparatus used for a "non-complex" ablation, these procedures often make use of sophisticated computer mapping systems to localize the source of the abnormal rhythm and to direct delivery of ablation lesions.
Surgical Procedures: Pacemaker and Defibrillator Implantation and Follow Up[edit | edit source]
- Implantation of single and dual chamber pacemakers and defibrillators
- Implantation of "biventricular" pacemakers and defibrillators for patients with congestive heart failure
- Implantation of loop recorders (implanted ECG recorders for long term monitoring of ECG to allow for diagnosis of an arrhythmia)
- Clinical follow up and reprogramming of implanted devices
Abnormalities in Cardiac Electrophysiology[edit | edit source]
- Mechanism of Arrhythmias
- Diseases of the Conduction System and Bradyarrhythmias
- Narrow Complex Tachycardias
- Wide Complex Tachycardias
- Ventricular Tachyarrhythmias, Cardiac Arrest and Sudden Cardiac Death
- Arrhythmias in Pregnancy
Treatment Modalities for Arrhythmia[edit | edit source]
- Antiarrhythmic Medications
- Indications for Pacemakers
- Indications for an ICD
- Cardiac Resynchronization Therapy
- Catheter ablation
See also[edit | edit source]
- Clinical cardiac electrophysiology
- Electrical conduction system of the heart
- Electrocardiogram (EKG)
- Basic Principles of ECG Interpretation
- Electrophysiologic study
- Cardiology
- Cardiac arrhythmia
External links[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 David E. Mohrman, L. J. (2010). Cardiovascular Physiology, 7e. The McGraw-Hill Companies, Inc.
- ↑ Kim E. Barrett, S. B. (2012). Ganong’s Review of Medical Physiology, 24e . The McGraw-Hill Companies, Inc.
- ↑ 3.0 3.1 Olson, E. N. (2004). A decade of discoveries in cardiac biology. Nature Medicine, 467 - 474.
- ↑ 4.0 4.1 Valentin Fuster, R. A. (2011). Hurst's The Heart, 13e. The McGraw-Hill Companies, Inc.
- ↑ Kim E. Barrett, S. B. (2012). Ganong’s Review of Medical Physiology, 24e . The McGraw-Hill Companies, Inc.
Licensed under CC BY-SA 3.0 | Source: https://www.wikidoc.org/index.php/Cardiac_electrophysiology36 views | Status: cached on October 03 2025 20:05:17↧ Download this article as ZWI file
 KSF
KSF