Cefaclor
 From Wikidoc - Reading time: 13 min
From Wikidoc - Reading time: 13 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cefaclor is an antibiotic that is FDA approved for the treatment of otitis media, lower respiratory tract infections, pharyngitis, tonsillitis, Urinary tract infections, skin structure infections. Common adverse reactions include diarrhea,nervousness, insomnia, confusion, hypertonia, dizziness,asthenia, edema , dyspnea, paresthesias, syncope, hypotension.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Cefaclor is indicated in the treatment of the following infections when caused by susceptible strains of the designated microorganisms:
- Otitis media caused by Streptococcus pneumoniae, Haemophilus influenzae, staphylococci, and Streptococcus pyogenes
Note: β-lactamase-negative, ampicillin-resistant (BLNAR) strains of Haemophilus influenzae should be considered resistant to cefaclor despite apparent in vitro susceptibility of some BLNAR strains.
- Lower respiratory tract infections, including pneumonia caused by Streptococcus pneumoniae, Haemophilus influenzae, and Streptococcus pyogenes.
Note: β-lactamase-negative, ampicillin-resistant (BLNAR) strains of Haemophilus influenzae should be considered resistant to cefaclor despite apparent in vitro susceptibility of some BLNAR strains.
- Pharyngitis and Tonsillitis, caused by Streptococcus pyogenes
Note: Penicillin is the usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever. Cefaclor is generally effective in the eradication of streptococci from the nasopharynx; however, substantial data establishing the efficacy of cefaclor in the subsequent prevention of rheumatic fever are not available at present.
- Urinary tract infections, including pyelonephritis and cystitis, caused by Escherichia coli, Proteus mirabilis, Klebsiella spp., and coagulase-negative staphylococci
- Skin and skin structure infections caused by Staphylococcus aureus and Streptococcus pyogenes
- Appropriate culture and susceptibility studies should be performed to determine susceptibility of the causative organism to cefaclor.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefaclor and other antibacterial drugs, cefaclor should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage
- The usual adult dosage is 250 mg every 8 hours. For more severe infections (such as pneumonia) or those caused by less susceptible organisms, doses may be doubled.
- Cefaclor may be administered in the presence of impaired renal function. Under such a condition, the dosage usually is unchanged.
- In the treatment of β-hemolytic streptococcal infections, a therapeutic dosage of cefaclor should be administered for at least 10 days.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cefaclor in adult patients.
Non–Guideline-Supported Use
Indications
Gonorrhea[1]
- Dosing
- A loading dose of 1 gram of CEFACLOR followed by 500 mg twice daily for 2 days
- Sinusitis[2]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Cefaclor is indicated in the treatment of the following infections when caused by susceptible strains of the designated microorganisms:
- Otitis media caused by Streptococcus pneumoniae, Haemophilus influenzae, staphylococci, and Streptococcus pyogenes
Note: β-lactamase-negative, ampicillin-resistant (BLNAR) strains of Haemophilus influenzae should be considered resistant to cefaclor despite apparent in vitro susceptibility of some BLNAR strains.
- Lower respiratory tract infections, including pneumonia caused by Streptococcus pneumoniae, Haemophilus influenzae, and Streptococcus pyogenes.
Note: β-lactamase-negative, ampicillin-resistant (BLNAR) strains of Haemophilus influenzae should be considered resistant to cefaclor despite apparent in vitro susceptibility of some BLNAR strains.
- Pharyngitis and Tonsillitis, caused by Streptococcus pyogenes
Note: Penicillin is the usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever. Cefaclor is generally effective in the eradication of streptococci from the nasopharynx; however, substantial data establishing the efficacy of cefaclor in the subsequent prevention of rheumatic fever are not available at present.
- Urinary tract infections, including pyelonephritis and cystitis, caused by Escherichia coli, Proteus mirabilis, Klebsiella spp., and coagulase-negative staphylococci
- Skin and skin structure infections caused by Staphylococcus aureus and Streptococcus pyogenes
- Appropriate culture and susceptibility studies should be performed to determine susceptibility of the causative organism to cefaclor.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefaclor and other antibacterial drugs, cefaclor should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage
- The usual recommended daily dosage for pediatric patients is 20 mg/kg/day in divided doses every 8 hours. In more serious infections, otitis media, and infections caused by less susceptible organisms, 40 mg/kg/day are recommended, with a maximum dosage of 1 g/day.
- Cefaclor may be administered in the presence of impaired renal function. Under such a condition, the dosage usually is unchanged.
- In the treatment of β-hemolytic streptococcal infections, a therapeutic dosage of cefaclor should be administered for at least 10 days.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cefaclor in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefaclor in pediatric patients.
Contraindications
- Cefaclor is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
Warnings
- BEFORE THERAPY WITH CEFACLOR IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFACLOR, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG β-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY.
- IF AN ALLERGIC REACTION TO CEFACLOR OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
- Antibiotics, including cefaclor, should be administered cautiously to any patient who has demonstrated some form of allergy, particularly to drugs.
- Pseudomembranous colitis has been reported with nearly all antibacterial agents, including cefaclor, and has ranged in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
- Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of antibiotic-associated colitis.
- After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation and treatment with an antibacterial drug effective against C. difficile.
PRECAUTIONS
General
- Prescribing cefaclor in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
- Prolonged use of cefaclor may result in the overgrowth of non susceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
- Positive direct Coombs’ tests have been reported during treatment with the cephalosporin antibiotics. It should be recognized that a positive Coombs’ test may be due to the drug, e.g., in hematologic studies or in transfusion cross-matching procedures when antiglobulin tests are performed on the minor side or in Coombs’ testing of newborns whose mothers have received cephalosporin antibiotics before parturition.
- Cefaclor should be administered with caution in the presence of markedly impaired renal function. Since the half-life of cefaclor in anuria is 2.3 to 2.8 hours, dosage adjustments for patients with moderate or severe renal impairment are usually not required. Clinical experience with cefaclor under such conditions is limited; therefore, careful clinical observation and laboratory studies should be made.
- As with other β-lactam antibiotics, the renal excretion of cefaclor is inhibited by probenecid. Antibiotics, including cephalosporins, should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Adverse Reactions
Clinical Trials Experience
Adverse effects considered to be related to therapy with cefaclor are listed below:
Hypersensitivity reactions have been reported in about 1.5% of patients and include morbilliform eruptions (1 in 100). Pruritus, urticaria, and positive Coombs’ tests each occur in less than 1 in 200 patients.
- Cases of serum-sickness-like reactions have been reported with the use of cefaclor. These are characterized by findings of erythema multiforme, rashes, and other skin manifestations accompanied by arthritis/arthralgia, with or without fever, and differ from classic serum sickness in that there is infrequently associated lymphadenopathy and proteinuria, no circulating immune complexes, and no evidence to date of sequelae of the reaction. Occasionally, solitary symptoms may occur, but do not represent a serum-sickness-like reaction. While further investigation is ongoing, serum-sickness-like reactions appear to be due to hypersensitivity and more often occur during or following a second (or subsequent) course of therapy with cefaclor. Such reactions have been reported more frequently in pediatric ptients than in adults with an overall occurrence ranging from 1 in 200 (0.5%) in one focused trial to 2 in 8,346 (0.024%) in overall clinical trials (with an incidence in pediatric patients in clinical trials of 0.055%) to 1 in 38,000 (0.003%) in spontaneous event reports. Signs and symptoms usually occur a few days after initiation of therapy and subside within a few days after cessation of therapy; occasionally these reactions have resulted in hospitalization, usually of short duration (median hospitalization = 2 to 3 days, based on postmarketing surveillance studies). In those requiring hospitalization, the symptoms have ranged from mild to severe at the time of admission with more of the severe reactions occurring in pediatric patients. Antihistamines and glucocorticoids appear to enhance resolution of the signs and symptoms. No serious sequelae have been reported.
- More severe hypersensitivity reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and anaphylaxis have been reported rarely. Anaphylactoid events may be manifested by solitary symptoms, including angioedema, asthenia, edema (including face and limbs), dyspnea, paresthesias, syncope, hypotension, or vasodilatation. Anaphylaxis may be more common in patients with a history of penicillin allergy.
- Rarely, hypersensitivity symptoms may persist for several months.
Gastrointestinal symptoms occur in about 2.5% of patients and include diarrhea (1 in 70).
- Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment. Nausea and vomiting have been reported rarely. As with some penicillins and some other cephalosporins, transient hepatitis and cholestatic jaundice have been reported rarely.
- Other effects considered related to therapy included eosinophilia (1 in 50 patients), genital pruritus , moniliasis or vaginitis (about 1 in 50 patients), and, rarely, thrombocytopenia or reversible interstitial nephritis.
Causal Relationship Uncertain–
CNS–Rarely, reversible hyperactivity, agitation, nervousness, insomnia, confusion, hypertonia, dizziness, hallucinations, and somnolence have been reported.
Transitory abnormalities in clinical laboratory test results have been reported. Although they were of uncertain etiology, they are listed below to serve as alerting information for the physician.
Hepatic–Slight elevations of AST, ALT, or alkaline phosphatase values (1 in 40).
Hematopoietic–As has also been reported with other β-lactam antibiotics, transient lymphocytosis, leukopenia, and, rarely, hemolytic anemia, aplastic anemia, agranulocytosis, and reversible neutropenia of possible clinical significance.
There have been rare reports of increased prothrombin time with or without clinical bleeding in patients receiving cefaclor and warfarin concomitantly.
Renal– Slight elevations in BUN or serum creatinine (less than 1 in 500) or abnormal urinalysis (less than 1 in 200).
Cephalosporin-class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with cefaclor, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics: fever, abdominal pain, superinfection, renal dysfunction, toxic nephropathy, hemorrhage, false positive test for urinary glucose, elevated bilirubin, elevated LDH, and pancytopenia.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Cefaclor in the drug label.
Drug Interactions
- There have been reports of increased anticoagulant effect when cefaclor and oral anticoagulants were administered concomitantly.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy–Teratogenic Effects–Pregnancy Category B
- Reproduction studies have been performed in mice and rats at doses up to 12 times the human dose and in ferrets given 3 times the maximum human dose and have revealed no harm to the fetus due to cefaclor. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cefaclor in women who are pregnant.
Labor and Delivery
- The effect of cefaclor on labor and delivery is unknown.
Nursing Mothers
- Small amounts of cefaclor have been detected in mother’s milk following administration of single 500 mg doses. Average levels were 0.18, 0.20, 0.21, and 0.16 mcg/mL at 2, 3, 4, and 5 hours respectively. Trace amounts were detected at 1 hour. The effect on nursing infants is not known. Caution should be exercised when cefaclor is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness of this product for use in infants less than 1 month of age have not been established.
Geriatic Use
- Of the 3703 patients in clinical studies of cefaclor, 594 (16.0%) were 65 and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Cefaclor with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cefaclor with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cefaclor in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cefaclor in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cefaclor in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cefaclor in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Cefaclor in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Cefaclor in the drug label.
Overdosage
Signs and Symptoms –The toxic symptoms following an overdose of cefaclor may include nausea, vomiting, epigastric distress, and diarrhea. The severity of the epigastric distress and the diarrhea are dose related. If other symptoms are present, it is probable that they are secondary to an underlying disease state, an allergic reaction, or the effects of other intoxication.
Treatment–To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
Unless 5 times the normal dose of cefaclor has been ingested, gastrointestinal decontamination will not be necessary.
Protect the patient’s airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient’s vital signs, blood gases, serum electrolytes, etc. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient’s airway when employing gastric emptying or charcoal.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of cefaclor.
Pharmacology
| |
Cefaclor
| |
| Systematic (IUPAC) name | |
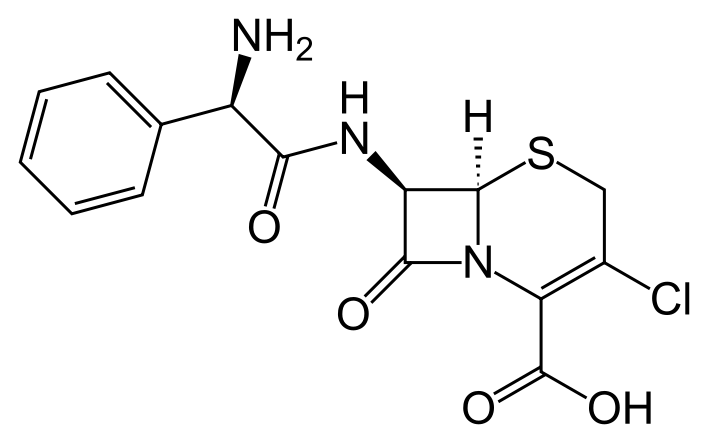
| (6R,7R)-7-{[(2R)-2-amino-2-phenylacetyl]amino}- 3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene- 2-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | J01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 367.808 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | Well absorbed, independent of food intake |
| Metabolism | 15% to 40% |
| Half life | 0.6 to 0.9 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral |
Mechanism of Action
There is limited information regarding Cefaclor Mechanism of Action in the drug label.
Structure
- Cefaclor is a semisynthetic cephalosporin antibiotic for oral administration. It is chemically designated as 3-chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate. The molecular formula for cefaclor is C15H14ClN3O4S•H2O and the molecular weight is 385.82.
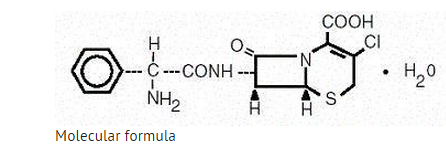
- Each capsule contains cefaclor monohydrate equivalent to 250 mg (0.68 mmol) or 500 mg (1.36 mmol) anhydrous cefaclor. The capsules also contain black iron oxide, croscarmellose sodium, FD&C Red No. 3, FD&C Blue No. 2, gelatin, magnesium stearate, corn starch, and titanium dioxide.
- The color of the capsule powder is white to off white.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Cefaclor in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Cefaclor in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Studies have not been performed to determine potential for carcinogenicity, mutagenicity, or impairment of fertility.
Clinical Studies
There is limited information regarding Clinical Studies of Cefaclor in the drug label.
How Supplied
Capsules:
- Cefaclor Capsules, USP 250 mg: opaque purple and white hard gelatin capsules imprinted with "West Ward 985" in bottles of 15 and bottles of 100.
- Cefaclor Capsules, USP 500 mg: opaque purple and gray hard gelatin capsules imprinted with "West Ward 986" in bottles of 15 and bottles of 100.
Storage
Store bottles at 20° to 25°C (68° to 77° F).
Images
Drug Images
{{#ask: Page Name::Cefaclor |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

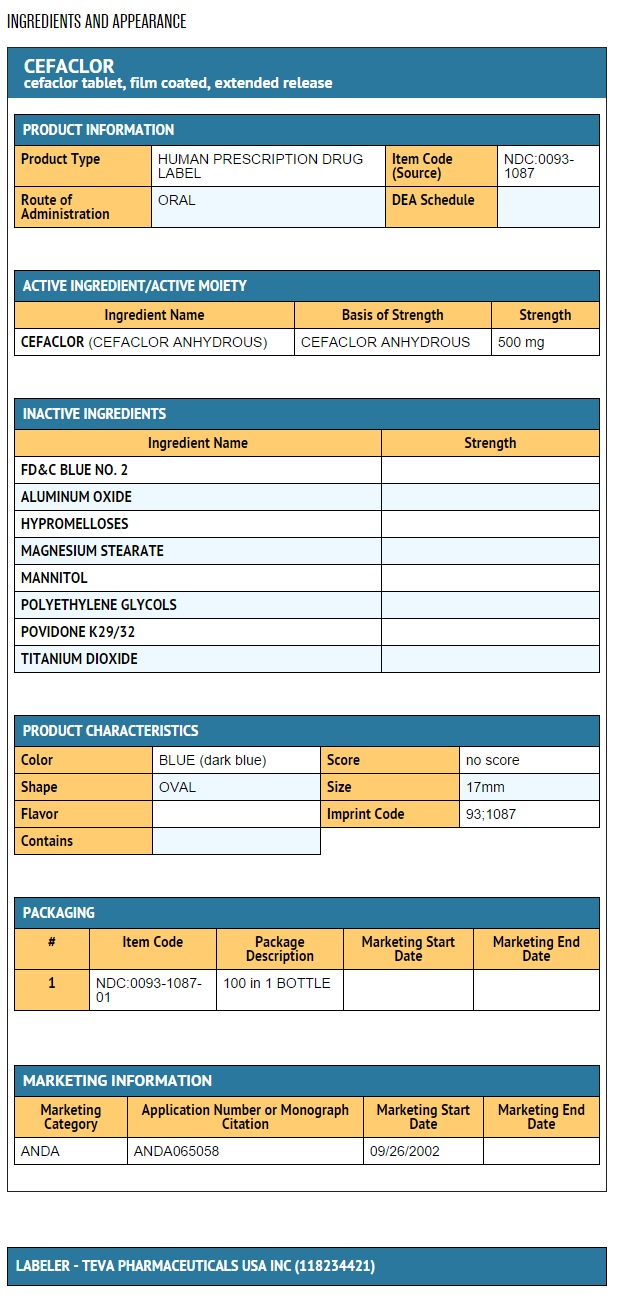
{{#ask: Label Page::Cefaclor |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be counseled that antibacterial drugs including cefaclor should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefaclor is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefaclor or other antibacterial drugs in the future.
Precautions with Alcohol
- Alcohol-Cefaclor interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
CEFACLOR
Look-Alike Drug Names
There is limited information regarding Cefaclor Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Spagna VA, Perkins RL, Prior RB (1979). "Successful treatment with cefaclor of gonococcal urethritis in men". Sex Transm Dis. 6 (3): 211–3. PMID 116373.
- ↑ Snow V, Mottur-Pilson C, Hickner JM, American Academy of Family Physicians. American College of Physicians-American Society of Internal Medicine. Centers for Disease Control; et al. (2001). "Principles of appropriate antibiotic use for acute sinusitis in adults". Ann Intern Med. 134 (6): 495–7. PMID 11255527.
{{#subobject:
|Page Name=Cefaclor |Pill Name=Cefaclor_NDC_00931087.jpg |Drug Name=Cefaclor |Pill Ingred=CEFACLOR[CEFACLOR ANHYDROUS]|+sep=; |Pill Imprint=93;1087 |Pill Dosage=500 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=17 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00931087
}}
{{#subobject:
|Label Page=Cefaclor |Label Name=Cefaclor fig.jpg
}}
{{#subobject:
|Label Page=Cefaclor |Label Name=Cefaclor ingredients and appearance.png
}}
 KSF
KSF