Dementia with Lewy bodies
 From Wikidoc - Reading time: 21 min
From Wikidoc - Reading time: 21 min
For patient information, click here
| Dementia with Lewy bodies | |
| ICD-10 | G31.8 |
|---|---|
| ICD-9 | 331.82 |
| DiseasesDB | 3800 |
| MeSH | D020961 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yazan Daaboul, M.D.; Kiran Singh, M.D. [2]
Synonyms and keywords: Lewy Body dementia (LBD), Diffuse Lewy Body disease (DLBD), Cortical Lewy Body disease (CLBD), and Senile dementia of Lewy type
Overview[edit | edit source]
Dementia with Lewy bodies (DLB) is the second most common cause of dementia following Alzheimer's disease. It usually affects patients aged > 50 years. The hallmark of DLB is the presence of Lewy bodies, which are intracytoplasmic inclusion bodies that contain synuclein proteins located in the cortical and subcortical neurons. However, these findings are not specific to DLB and may similarly be present in other neurodegenerative diseases. Although the majority of DLB cases are due to sporadic development, DLB may be familial, suggesting a genetic predisposition. Clinically, DLB is characterized by the development of a triad of features, namely cognitive, neurological, and psychiatric symptoms. The diagnosis of DLB is difficult, given the absence of distinguishing features on imaging and neuropathology. Instead, physicians should rely exclusively on the presence of core and suggestive clinical features that may distinguish DLB from other neurodenegerative disorders that cause dementia and symptoms of parkinsonism, such as Alzheimer's disease (AD) and Parkinson's disease with dementia (PDD). Distinguishing features of DLB include the presence of dementia followed by early parkinsonism symptoms within less than 1 year of symptoms-onset, vivid and detailed visual hallucinations, depressive symptoms, REM sleep disorders, neuroleptic sensitivity, early-onset postural instability and falls, and prominent visuospatial and verbal learning impairment. There is currently no cure for DLB, but management is generally aimed at improving quality of life via pharmacologic and non-pharmacologic interventions. Cholinesterase inhibitors, such as rivastigmine and memantine, have demonstrated efficacy in the treatment of behavioral symptoms among patients with DLB, and they are the first-line pharmacologic agents for the management of DLB. The prognosis of DLB is poor with a median survival time comparable to that of AD (3-8 years), but more aggressive forms have also been described.
Historical Perspective[edit | edit source]
- Lewy body dementia (LBD) was named after Frederich Heinrich Lewy, a German-American neurologist who discovered the characteristic intracytoplasmic inclusions in 1912.[1][2]
- In 1961, Okazaki suggested that the presence of cortical Lewy bodies in brain tissue was associated with the development of dementia.[1]
- In 1984, Kosaka and colleagues hypothesized that the presence of Lewy bodies may correspond to a new disease entity, which was eventually named "diffuse Lewy body disease".[3] The disease name was then changed in 1996 at the First International Workshop of the Consortium on Dementia with Lewy Bodies to become "dementia with Lewy bodies" (DLB).[4]
- DLB was not considered a diagnosis of dementia in the first 4 versions of the Diagnostic and Statistical Manual (DSM) for Mental Disorders. In 2013, DSM-5 incorporated DLB as a differential diagnosis for dementia.[5][2]
Pathophysiology[edit | edit source]
Genetics[edit | edit source]
Familial predisposition to DLB has been frequently described in observational studies, suggesting the role of genetics in the development of DLB. To date, genetic mutations that are exclusively implicated in DLB have not been described; genetic determinants of DLB have also been frequently reported in Parkinson's disease.
SNCA Gene[edit | edit source]
Predisposition to DLB may be caused by autosomal dominant genetic mutations of the Synuclein gene family.[6][7] Synuclein proteins are members of a pre-synpatic protein family located in the central and peripheral nervous systems. Although the majority of reports describe defects of the α-synuclein protein overexpression in DLB, the β- and γ-synuclein protein variants have also been associated with development of DLB. Mutations of α-synuclein (SNCA) gene have been classically associated with Parkinson's disease with dementia (PDD), but triplication defects and mutations of the E46K and A53T loci within the SNCA gene have been described in patients with DLB.[8][9][10][10]
Other Genes[edit | edit source]
Mutations of the following genes are also associated with the development of DLB are:
- LRRK2[11][12]
- Glucocerebrosidase (GBA) gene mutation among patients with Gaucher's disease and their relatives[13]
- V70M[10]
- P123H[10]
- 2q35-q36 locus on chromosome 2 (adjacent to PARK11 locus whose mutation is associated with PD)[14]
Pathology[edit | edit source]
The hallmark of dementia with Lewy bodies (DLB) is the presence of Lewy bodies (LB) that are accompanied by dystropic Lewy neurite (LN) in cortical, brainstem, and limbic system neurons. Lewy bodies are eosinophilic, filamentous, intracytoplasmic inclusion bodies composed of the following components:[15][16][17][18][19][20]
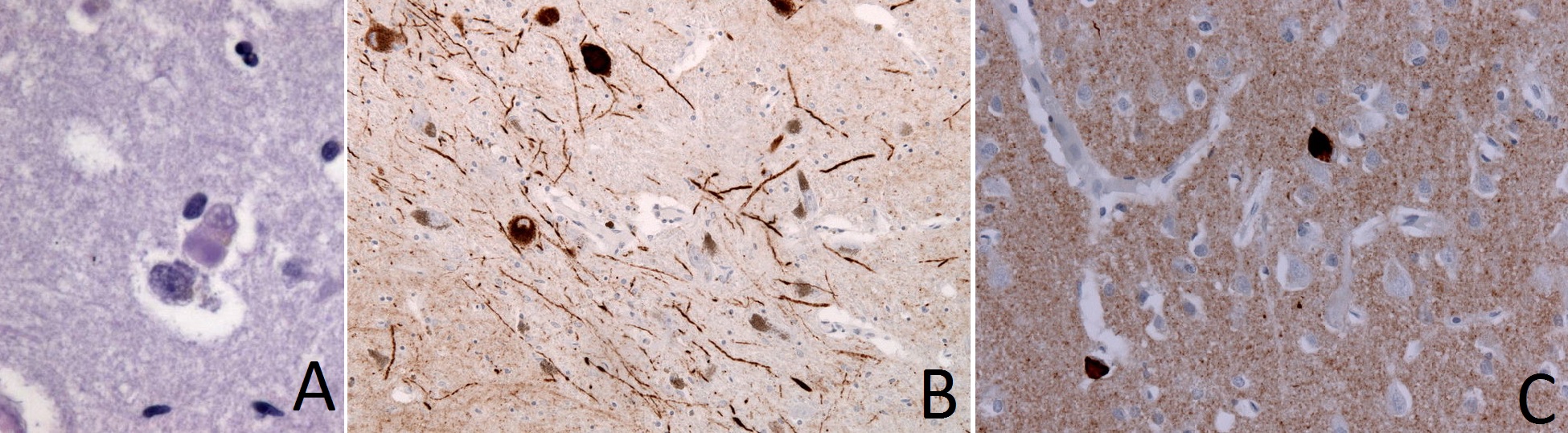
Figure: A. Lewy bodies (synuclein-positive, eosinophilic, neuronal inclusions) in brain tissue of patient with dementia with Lewy bodies (DLB); B. Immunohistochemical staining of Lewy Neurites; C. Immunhistochemical staining of frontal cortex in DLB demonstrates alpha-Synuclein positive inclusions (Images courtesy of user:Jensflorian from http://commons.wikimedia.org/ licensed under the Creative Commons Attribution-Share Alike 3.0 Unported under the terms of GNU Free Documentation License)
Classically, Lewy bodies in DLB are initially present in the amygdala. As the disease advances, these bodies spread to the limbic cortex and then to the neocortex. These findings may explain the predominance of dementia in patients with early DLB and the consequent development of parkinsonism symptoms.[21] The clinical features of DLB are directly associated with the severity of Lewy-related pathology. In turn, severity is measured by the pattern of regional involvement of Lewy bodies rather than the number of Lewy bodies.[22]
Classification[edit | edit source]
DLB may be classified according to the regional involvement of the brain tissue based upon the presence of Lewy bodies:
- Diffuse neocortical DLB
- Brainstem predominant DLB
- Limbic/transitional DLB
Differential Diagnosis[edit | edit source]
Dementia with Lewy bodies (DLB) should be distinguished from other disorders that cause memory impairment, recurrent hallucinations, and Parkinsonism. The most important differential diagnoses of DLB are Alzheimer's disease (AD) and Parkinson's disease with dementia (PDD) due to the overlapping clinical features with absence of distinguishing radiographic or neuropathological features across the 3 diseases. Generally, the distinction between the 3 disease is exclusively clinical based on key findings during history-taking and physical examination.
Alzheimer's Disease (AD) and Parkinsons's Disease with Dementia (PDD)[edit | edit source]
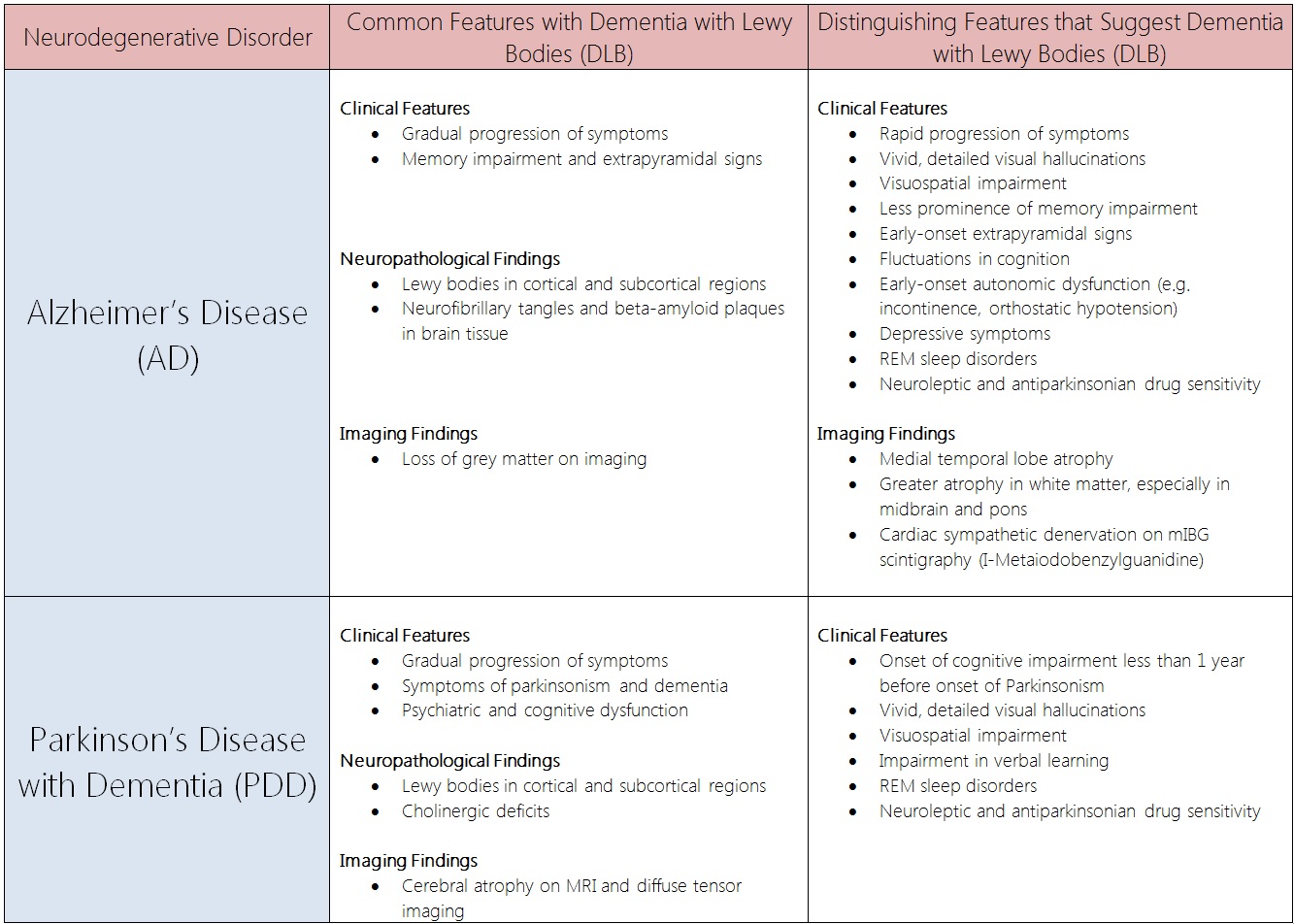
Comparison Table: Dementia with Lewy Bodies (DLB) vs. Alzheimer's Disease (AD) and Parkinson's Disease with Dementia (PDD)[23][24][25][26][27][28][29][30][31][32][33][34][35][36][31][37][38][39][40][41]
Other Neurodegenerative Disorders[edit | edit source]
Other neurodegenerative disorders may also be considered in the differential diagnosis of DLB[23]:
- Parkinson-plus syndromes (such as progressive supranuclear palsy, corticobasal degeneration, and multiple system atrophy)
- Cortical basal ganglionic degeneration
- Frontotemporal dementia (FTD or Pick's disease)
- Hydrocephalus
- Prion-associated neurodegenerative diseases (eg. Creutzfeldt-Jakob disease)
- Substance abuse and drug-induced neuropsychiatric symptoms
- The following table outlines the main findings of the diseases which must be differentiated from dementia with Lewy bodies as they may share common characteristics of cognitive impairment:[42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57]
| Cause of dementia | Clinical features | Associated features | Nature of progression | Histopathological findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive impairment | ||||||||||||||
| Recall | Recollection | Cue requirement for recall | Infirngement of thoughts | Semantic memory | Procedural memory | Working memory | Awareness | Attention | Executive functioning issues | Visuo-spatial skills | ||||
| Alzheimer's disease | +++
(Slow cognitive and functional decline with early loss of awareness) |
+++ | Not helpful | +++ | ++ | - | ++ | +++ | ++ | ++ | ++ |
|
Has the following clinical stages:
|
|
| Lewy body dementia | ++ | - | Helpful | +++ | + | + | +++ | + | +++ | +++ | +++ |
|
| |
| Frontotemporal lobar degeneration | +/- | - | Helpful | +++ | + | - | +++ | +++ | ++ | +++ | - |
|
|
|
| Vascular dementia | + (Dysexecutive syndrome) | - | Helpful | + | + | + | ++ | - | ++ | +++ | + |
|
|
|
Epidemiology and Demographics[edit | edit source]
DLB is the 2nd most common cause of dementia following Alzheimer's disease (AD) and is the underlying etiology of approximately 1.7-30.5% of dementia cases. DLB generally affects patients older than 50 years of age and has a slight male predilection.
Incidence[edit | edit source]
- In the general population, the annual incidence of DLB is 100 per 100,000 persons.[58]
- Among patients with dementia, the annual incidence of DLB is 3,200 - 5,000 per 100,000 persons.[58]
Prevalence[edit | edit source]
- In the general population, the prevalence of DLB is approximately 5,000 per 100,000 persons.[59][60][61][62][63][64]
- Among patients with dementia, the prevalence of DLB ranges between 1,700 to 30,500 per 100,000 persons.[59][60][61][62][63][64]
Age[edit | edit source]
- Age at diagnosis is usually between 50 - 83 years.[65]
Gender[edit | edit source]
- DLB has a slight male predilection.[65]
Risk Factors[edit | edit source]
- Familial predisposition to DLB has been frequently described in observational studies, suggesting the role of genetics in the development of DLB.[66]
Natural History[edit | edit source]
Presenting Symptoms[edit | edit source]
The presenting symptoms of DLB vary and may include any of the following:[67][2][65]
- Memory impairment (40 - 100%)
- Parkinsonism (10-78%)
- Depression (7-75%)
- Visual hallucinations (11-64%)
- Falls (10-38%)
- Problem-solving difficulty (33%)
- Stiffness (25%)
Common Symptoms of Advanced Disease[edit | edit source]
- 100% of patients with DLB eventually develop dementia early during the course of the disease.[65]
- Similarly, the development of parkinsonism symptoms has also been reported in up to 50-100% of patients.[65]
- In advanced disease, DLB symptoms eventually include cognitive fluctuation (45-90%), depression (12-89%), visual hallucinations (13-80%), and falls (22-50%).[65]
Prognosis[edit | edit source]
- In a minority of patients, the disease progresses aggressively, and patients may die within 1-2 years of symptoms-onset.[65]
Clinical Features[edit | edit source]
Dementia with Lewy bodies (DLB) is characterized by the triad of progressive cognitive impairment, parkinsonism, and neuropsychiatric disturbances. The diagnosis of the DLB is usually made clinically based on history-taking and findings on physical examination in the absence of metabolic, vascular, and degenerative disoders. Unfortunately, the clinical features of DLB frequently overlap with other neurodegenerative diseases, namely Alzheimer's disease and Parkinson's disease, and some experts consider DLB to be part of the Alzheimer's/Parkinsonism spectrum.
Clinical Pearls: Distinguishing Features[edit | edit source]
There are several distinguishing clinical features that suggest the diagnosis of DLB:
- Dementia in DLB precedes parkinsonism symptoms.
- The progression of signs and symptoms in DLB is relatively rapid compared to other neurodegenerative diseases. The occurrence of parkinsonism is frequently observed within less than one year of onset of dementia. Classically, patients with DLB report postural instability (eg. frequent falls and bone fractures) early in the disease compared to the delayed onset of postural instability observed in Parkinson's disease.
- Hallucinations in DLB are frequently visual, well-formed, and thoroughly described, compared to auditory hallucinations typically observed in schizophrenia or tactile hallucinations commonly observed in drug-induced disorders and delirium.
- Postural instability in DLB manifests early. Unlike patients with Parkinson's disease, patients with DLB report frequent falls that may be present as early as a few months after onset of symptoms.[26]
- Sleep disturbances in DLB are often due to REM sleep disorder. While other neurodegenerative diseases are associated with sleep disorders, REM sleep disorders have been frequently associated with DLB.
- Sensitivity to neuroleptic agents and to antiparkinsonian drugs. As many patients with DLB are diagnosed with psychosis or Parkinson's disease due to the presence of overlapping features, symptoms in DLB paradoxically exacerbate upon administration of neuroleptics and antiparkinsonian drugs. Patients often experience worse psychotic symptoms (antiparkinsonian drugs) and parkinsonism symptoms (neuroleptics).[69][26]
Main Clinical Features[70][edit | edit source]
Motor[edit | edit source]
Cognitive[edit | edit source]
- Cognitive impairment (eg. executive dysfunction, attention deficit, visuospatial dysfunction, impaired language, impaired calculations)
- Cognitive fluctuations
Psychiatric[edit | edit source]
- Hallucinations (mostly visual)
- Delusions
- Depressive mood
Dysautonomic[edit | edit source]
Less Common Features[edit | edit source]
Motor[edit | edit source]
Psychiatric[edit | edit source]
Dysautonomic[edit | edit source]
Sleep Disorders[edit | edit source]
- REM sleep behavior disorder
- Insomnia
- Excessive daytime sleepiness
- Restless legs
- Periodic limb movements
- Sleep apnea
- Nightmares
Others[edit | edit source]
Diagnostic Criteria[edit | edit source]
DSM-V Diagnostic Criteria for Major or Mild Neurocognitive Disorder With Lewy Bodies[66][edit | edit source]
| “ |
AND
AND
For probable major or mild neurocognitive disorder with Lewy bodies, the individual has two core features, or one suggestive feature with one or more core features. For possible major or mild neurocognitive disorder with Lewy bodies, the individual has only one core feature, or one or more suggestive features.
AND
|
” |
Management[edit | edit source]
Management Goals[edit | edit source]
Management consists of a 4-step approach[71]:
- Establish an accurate and timely diagnosis
- Identify severity and nature of clinical features
- Provide non-pharmacologic interventions
- Administer pharmacologic therapy
Non-pharmacologic Interventions[edit | edit source]
Non-pharmacologic interventions are the mainstay of DLB management, given the lack of efficacy and at at times, paradoxical responses induced by some pharmacologic agents.[71]
- Education of patients and caregivers
- Mobility aid and physiotherapy
Pharmacologic Interventions[edit | edit source]
Cholinesterase Inhibitors[edit | edit source]
- Cholinesterase inhibitors are the first-line pharmacologic agents to treat cognitive and psychiatric symptoms of patients with DLB.[72]
- Rivastigmine: The efficacy of rivastigmine in DLB was studied in a multi-center, randomized, controlled trial (n=120).[72]
- Memantine: The efficacy of memantine in DLB was studied in a randomized, double-blind, placebo-controlled trial (n=34).[73]
- The trial compared the baseline Alzheimer's disease cooperative study (ADCS)-clinical global impression of change scores to the scores at 24 weeks.[73]
- Memantine improved global clinical status and behavioral symptoms of patients with mild to moderate DLB.[73]
- The recommended dose in the trial was memantine 20 mg once daily.[73]
- The trial compared the baseline Alzheimer's disease cooperative study (ADCS)-clinical global impression of change scores to the scores at 24 weeks.[73]
- Donepezil, rivastigmine, and galantamine have demonstrated clinical efficacy among patients with DLB in open-label studies.[74][75][76][77]
- Despite efficacy, a minority of patients reported worsening symptoms of parkinsonism when administered cholinesterase inhibitors.[72][72]
Atypical Antipsychotics[edit | edit source]
- Based on expert-opinion, atypical antipsychotics are the second-line pharmacologic agents for management of symptoms of DLB.
- Antipsychotic agents are indicated only if cholinesterase inhibitors have proven ineffective.
Benzodiazepines[edit | edit source]
- Clonazepam has demonstrated efficacy in the treatment of REM sleep disorder among 14 patients with DLB.[78]
Selective Serotonine Receptor Inhibitors (SSRI)[edit | edit source]
- SSRI are recommended as first-line agents for the management of depression associated with DLB.
Dopamine Agonists[edit | edit source]
- Levodopa-carbidopa has demonstrated efficacy in the treatment of parkinsonism symptoms among one third of patients with DLB (n=14).[69]
- The trial assessed the Unified Parkinson's Disease Rating Scale, motor subsection (UPDRS III), finger tapping, and walking tests at baseline and after 6 months of therapy.[69]
- The mean daily dose in the trial was levodopa-carbidopa 323 mg.
- Younger patients were more responsive to levodopa-carbidopa therapy.[69]
- Generally, levodopa-carbidopa was well-tolerated, but a minority of patients experienced exacerbations of confusion.[69]
Other Pharmacologic Agents[edit | edit source]
- Neuroleptics (first generation antipsychotic agents) and antiparkinsonian agents are generally not recommended in patients with DLB, given the high rates of sensitivity to these drugs. When administered neuroleptics, patients often report worsening motor symptoms. When administered antiparkinsonian agents, some patients reported exacerbations of hallucinations.
References[edit | edit source]
- ↑ 1.0 1.1 OKAZAKI H, LIPKIN LE, ARONSON SM (1961). "Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion". J Neuropathol Exp Neurol. 20: 237–44. PMID 13730588.
- ↑ 2.0 2.1 2.2 Huang Y, Halliday G (2013). "Can we clinically diagnose dementia with Lewy bodies yet?". Transl Neurodegener. 2 (1): 4. doi:10.1186/2047-9158-2-4. PMC 3575256. PMID 23398715.
- ↑ Kosaka K, Yoshimura M, Ikeda K, Budka H (1984). "Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree--a new disease?". Clin Neuropathol. 3 (5): 185–92. PMID 6094067.
- ↑ McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA; et al. (1996). "Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop". Neurology. 47 (5): 1113–24. PMID 8909416.
- ↑ Regier DA, Kuhl EA, Kupfer DJ (2013). "The DSM-5: Classification and criteria changes". World Psychiatry. 12 (2): 92–8. doi:10.1002/wps.20050. PMC 3683251. PMID 23737408.
- ↑ Harding AJ, Das A, Kril JJ, Brooks WS, Duffy D, Halliday GM (2004). "Identification of families with cortical Lewy body disease". Am J Med Genet B Neuropsychiatr Genet. 128B (1): 118–22. doi:10.1002/ajmg.b.30014. PMID 15211643.
- ↑ Nishioka K, Wider C, Vilariño-Güell C, Soto-Ortolaza AI, Lincoln SJ, Kachergus JM; et al. (2010). "Association of alpha-, beta-, and gamma-Synuclein with diffuse lewy body disease". Arch Neurol. 67 (8): 970–5. doi:10.1001/archneurol.2010.177. PMID 20697047.
- ↑ Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I; et al. (2004). "The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia". Ann Neurol. 55 (2): 164–73. doi:10.1002/ana.10795. PMID 14755719.
- ↑ Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J; et al. (2003). "alpha-Synuclein locus triplication causes Parkinson's disease". Science. 302 (5646): 841. doi:10.1126/science.1090278. PMID 14593171.
- ↑ 10.0 10.1 10.2 10.3 Ohtake H, Limprasert P, Fan Y, Onodera O, Kakita A, Takahashi H; et al. (2004). "Beta-synuclein gene alterations in dementia with Lewy bodies". Neurology. 63 (5): 805–11. PMC 1808539. PMID 15365127.
- ↑ Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S; et al. (2004). "Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology". Neuron. 44 (4): 601–7. doi:10.1016/j.neuron.2004.11.005. PMID 15541309.
- ↑ Ross OA, Toft M, Whittle AJ, Johnson JL, Papapetropoulos S, Mash DC; et al. (2006). "Lrrk2 and Lewy body disease". Ann Neurol. 59 (2): 388–93. doi:10.1002/ana.20731. PMID 16437559.
- ↑ Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E (2004). "Parkinsonism among Gaucher disease carriers". J Med Genet. 41 (12): 937–40. doi:10.1136/jmg.2004.024455. PMC 1735652. PMID 15591280.
- ↑ Pankratz N, Nichols WC, Uniacke SK, Halter C, Rudolph A, Shults C; et al. (2003). "Significant linkage of Parkinson disease to chromosome 2q36-37". Am J Hum Genet. 72 (4): 1053–7. doi:10.1086/374383. PMC 1180337. PMID 12638082.
- ↑ Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, Bodi I; et al. (2009). "Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium". Acta Neuropathol. 117 (6): 635–52. doi:10.1007/s00401-009-0523-2. PMID 19330340.
- ↑ Braak H, Bohl JR, Müller CM, Rüb U, de Vos RA, Del Tredici K (2006). "Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered". Mov Disord. 21 (12): 2042–51. doi:10.1002/mds.21065. PMID 17078043.
- ↑ Ma SY, Ciliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ; et al. (1999). "Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra". J Comp Neurol. 409 (1): 25–37. PMID 10363709.
- ↑ Jellinger KA (2009). "A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders". Biochim Biophys Acta. 1792 (7): 730–40. doi:10.1016/j.bbadis.2008.07.006. PMID 18718530.
- ↑ Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H (2002). "Where does parkinson disease pathology begin in the brain?". J Neuropathol Exp Neurol. 61 (5): 413–26. PMID 12030260.
- ↑ den HARTOG JAGER WA, BETHLEM J (1960). "The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans". J Neurol Neurosurg Psychiatry. 23: 283–90. PMC 497426. PMID 13711997.
- ↑ Katsuse O, Iseki E, Marui W, Kosaka K (2003). "Developmental stages of cortical Lewy bodies and their relation to axonal transport blockage in brains of patients with dementia with Lewy bodies". J Neurol Sci. 211 (1–2): 29–35. PMID 12767494.
- ↑ Lippa CF, McKeith I (2003). "Dementia with Lewy bodies: improving diagnostic criteria". Neurology. 60 (10): 1571–2. PMID 12771241.
- ↑ 23.0 23.1 Morra LF, Donovick PJ (2014). "Clinical presentation and differential diagnosis of dementia with Lewy bodies: a review". Int J Geriatr Psychiatry. 29 (6): 569–76. doi:10.1002/gps.4039. PMID 24150834.
- ↑ Yoshizawa H, Vonsattel JP, Honig LS (2013). "Early neuropsychological discriminants for Lewy body disease: an autopsy series". J Neurol Neurosurg Psychiatry. 84 (12): 1326–30. doi:10.1136/jnnp-2012-304381. PMID 23308020.
- ↑ Kaur B, Harvey DJ, Decarli CS, Zhang L, Sabbagh MN, Olichney JM (2013). "Extrapyramidal signs by dementia severity in Alzheimer disease and dementia with Lewy bodies". Alzheimer Dis Assoc Disord. 27 (3): 226–32. doi:10.1097/WAD.0b013e31826f040d. PMC 3562426. PMID 23023095.
- ↑ 26.0 26.1 26.2 Zupancic M, Mahajan A, Handa K (2011). "Dementia with lewy bodies: diagnosis and management for primary care providers". Prim Care Companion CNS Disord. 13 (5). doi:10.4088/PCC.11r01190. PMC 3267516. PMID 22295275.
- ↑ Allan LM, Ballard CG, Allen J, Murray A, Davidson AW, McKeith IG; et al. (2007). "Autonomic dysfunction in dementia". J Neurol Neurosurg Psychiatry. 78 (7): 671–7. doi:10.1136/jnnp.2006.102343. PMC 2117678. PMID 17178816.
- ↑ Harding AJ, Broe GA, Halliday GM (2002). "Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe". Brain. 125 (Pt 2): 391–403. PMID 11844739.
- ↑ Dickson DW, Davies P, Mayeux R, Crystal H, Horoupian DS, Thompson A; et al. (1987). "Diffuse Lewy body disease. Neuropathological and biochemical studies of six patients". Acta Neuropathol. 75 (1): 8–15. PMID 3434218.
- ↑ Swerdlow RH, Newell KL (2012). ""Untangling" the relationship between Alzheimer disease and dementia with Lewy bodies". Neurology. 79 (19): 1938–9. doi:10.1212/WNL.0b013e3182735ecf. PMID 23035062.
- ↑ 31.0 31.1 Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard CG; et al. (2002). "Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry". Neuroimage. 17 (2): 618–30. PMID 12377138.
- ↑ Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE; et al. (2007). "Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease". Brain. 130 (Pt 3): 708–19. doi:10.1093/brain/awl388. PMC 2730778. PMID 17267521.
- ↑ Fujishiro H, Nakamura S, Kitazawa M, Sato K, Iseki E (2012). "Early detection of dementia with Lewy bodies in patients with amnestic mild cognitive impairment using 123I-MIBG cardiac scintigraphy". J Neurol Sci. 315 (1–2): 115–9. doi:10.1016/j.jns.2011.11.012. PMID 22129938.
- ↑ McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H; et al. (2005). "Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium". Neurology. 65 (12): 1863–72. doi:10.1212/01.wnl.0000187889.17253.b1. PMID 16237129.
- ↑ Calderon J, Perry RJ, Erzinclioglu SW, Berrios GE, Dening TR, Hodges JR (2001). "Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer's disease". J Neurol Neurosurg Psychiatry. 70 (2): 157–64. PMC 1737215. PMID 11160462.
- ↑ Guidi M, Paciaroni L, Paolini S, De Padova S, Scarpino O (2006). "Differences and similarities in the neuropsychological profile of dementia with Lewy bodies and Alzheimer's disease in the early stage". J Neurol Sci. 248 (1–2): 120–3. doi:10.1016/j.jns.2006.05.017. PMID 16784757.
- ↑ Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT (2004). "Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls". Brain. 127 (Pt 4): 791–800. doi:10.1093/brain/awh088. PMID 14749292.
- ↑ Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP (2003). "Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer's disease". J Neurol Neurosurg Psychiatry. 74 (9): 1215–20. PMC 1738667. PMID 12933921.
- ↑ Vernon AC, Ballard C, Modo M (2010). "Neuroimaging for Lewy body disease: is the in vivo molecular imaging of α-synuclein neuropathology required and feasible?". Brain Res Rev. 65 (1): 28–55. doi:10.1016/j.brainresrev.2010.05.006. PMID 20685363.
- ↑ Filoteo JV, Salmon DP, Schiehser DM, Kane AE, Hamilton JM, Rilling LM; et al. (2009). "Verbal learning and memory in patients with dementia with Lewy bodies or Parkinson's disease with dementia". J Clin Exp Neuropsychol. 31 (7): 823–34. doi:10.1080/13803390802572401. PMC 2935683. PMID 19221922.
- ↑ Mondon K, Gochard A, Marqué A, Armand A, Beauchamp D, Prunier C; et al. (2007). "Visual recognition memory differentiates dementia with Lewy bodies and Parkinson's disease dementia". J Neurol Neurosurg Psychiatry. 78 (7): 738–41. doi:10.1136/jnnp.2006.104257. PMC 2117680. PMID 17287240.
- ↑ Jellinger KA (2008). "The pathology of "vascular dementia": a critical update". J. Alzheimers Dis. 14 (1): 107–23. PMID 18525132.
- ↑ Murayama S (2008). "[Neuropathology of frontotemporal dementia]". Rinsho Shinkeigaku (in Japanese). 48 (11): 998. PMID 19198143.
- ↑ Hodges JR, Patterson K (1996). "Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study". J Int Neuropsychol Soc. 2 (6): 511–24. PMID 9375155.
- ↑ Hodges JR, Patterson K, Oxbury S, Funnell E (1992). "Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy". Brain. 115 ( Pt 6): 1783–806. PMID 1486461.
- ↑ "Dementia, Globalization and Contemporary Art".
- ↑ Helkala EL, Laulumaa V, Soininen H, Riekkinen PJ (1988). "Recall and recognition memory in patients with Alzheimer's and Parkinson's diseases". Ann. Neurol. 24 (2): 214–7. doi:10.1002/ana.410240207. PMID 3178177.
- ↑ Weintraub S, Wicklund AH, Salmon DP (2012). "The neuropsychological profile of Alzheimer disease". Cold Spring Harb Perspect Med. 2 (4): a006171. doi:10.1101/cshperspect.a006171. PMC 3312395. PMID 22474609.
- ↑ Goldman JG, Williams-Gray C, Barker RA, Duda JE, Galvin JE (2014). "The spectrum of cognitive impairment in Lewy body diseases". Mov. Disord. 29 (5): 608–21. doi:10.1002/mds.25866. PMC 4126402. PMID 24757110.
- ↑ Metzler-Baddeley C (2007). "A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer's disease and Parkinson's disease with dementia". Cortex. 43 (5): 583–600. PMID 17715794.
- ↑ Uversky VN (2008). "Alpha-synuclein misfolding and neurodegenerative diseases". Curr. Protein Pept. Sci. 9 (5): 507–40. PMID 18855701.
- ↑ Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE (2004). "Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function". Arch. Neurol. 61 (3): 378–84. doi:10.1001/archneur.61.3.378. PMID 15023815.
- ↑ Brion JP (1998). "Neurofibrillary tangles and Alzheimer's disease". Eur. Neurol. 40 (3): 130–40. PMID 9748670.
- ↑ Lee JS, Jung NY, Jang YK, Kim HJ, Seo SW, Lee J, Kim YJ, Lee JH, Kim BC, Park KW, Yoon SJ, Jeong JH, Kim SY, Kim SH, Kim EJ, Park KC, Knopman DS, Na DL (2017). "Prognosis of Patients with Behavioral Variant Frontotemporal Dementia Who have Focal Versus Diffuse Frontal Atrophy". J Clin Neurol. 13 (3): 234–242. doi:10.3988/jcn.2017.13.3.234. PMC 5532319. PMID 28748674.
- ↑ Pao WC, Dickson DW, Crook JE, Finch NA, Rademakers R, Graff-Radford NR (2011). "Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically". Alzheimer Dis Assoc Disord. 25 (4): 364–8. doi:10.1097/WAD.0b013e31820f8f50. PMC 3107353. PMID 21346515.
- ↑ Tsolaki M, Kokarida K, Iakovidou V, Stilopoulos E, Meimaris J, Kazis A (2001). "Extrapyramidal symptoms and signs in Alzheimer's disease: prevalence and correlation with the first symptom". Am J Alzheimers Dis Other Demen. 16 (5): 268–78. doi:10.1177/153331750101600512. PMID 11603162.
- ↑ McGuinness B, Barrett SL, Craig D, Lawson J, Passmore AP (2010). "Executive functioning in Alzheimer's disease and vascular dementia". Int J Geriatr Psychiatry. 25 (6): 562–8. doi:10.1002/gps.2375. PMID 19810010.
- ↑ 58.0 58.1 Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L (2002). "Incidence of AD may decline in the early 90s for men, later for women: The Cache County study". Neurology. 58 (2): 209–18. PMID 11805246.
- ↑ 59.0 59.1 de Silva HA, Gunatilake SB, Smith AD (2003). "Prevalence of dementia in a semi-urban population in Sri Lanka: report from a regional survey". Int J Geriatr Psychiatry. 18 (8): 711–5. doi:10.1002/gps.909. PMID 12891639.
- ↑ 60.0 60.1 Herrera E, Caramelli P, Silveira AS, Nitrini R (2002). "Epidemiologic survey of dementia in a community-dwelling Brazilian population". Alzheimer Dis Assoc Disord. 16 (2): 103–8. PMID 12040305.
- ↑ 61.0 61.1 Rahkonen T, Eloniemi-Sulkava U, Rissanen S, Vatanen A, Viramo P, Sulkava R (2003). "Dementia with Lewy bodies according to the consensus criteria in a general population aged 75 years or older". J Neurol Neurosurg Psychiatry. 74 (6): 720–4. PMC 1738491. PMID 12754338.
- ↑ 62.0 62.1 Stevens T, Livingston G, Kitchen G, Manela M, Walker Z, Katona C (2002). "Islington study of dementia subtypes in the community". Br J Psychiatry. 180: 270–6. PMID 11872521.
- ↑ 63.0 63.1 Yamada T, Hattori H, Miura A, Tanabe M, Yamori Y (2001). "Prevalence of Alzheimer's disease, vascular dementia and dementia with Lewy bodies in a Japanese population". Psychiatry Clin Neurosci. 55 (1): 21–5. doi:10.1046/j.1440-1819.2001.00779.x. PMID 11235852.
- ↑ 64.0 64.1 Yamada T, Kadekaru H, Matsumoto S, Inada H, Tanabe M, Moriguchi EH; et al. (2002). "Prevalence of dementia in the older Japanese-Brazilian population". Psychiatry Clin Neurosci. 56 (1): 71–5. doi:10.1046/j.1440-1819.2002.00931.x. PMID 11929573.
- ↑ 65.0 65.1 65.2 65.3 65.4 65.5 65.6 65.7 McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J; et al. (2004). "Dementia with Lewy bodies". Lancet Neurol. 3 (1): 19–28. PMID 14693108.
- ↑ 66.0 66.1 Diagnostic and statistical manual of mental disorders : DSM-5. Washington, D.C: American Psychiatric Association. 2013. ISBN 0890425558.
- ↑ Auning E, Rongve A, Fladby T, Booij J, Hortobágyi T, Siepel FJ; et al. (2011). "Early and presenting symptoms of dementia with lewy bodies". Dement Geriatr Cogn Disord. 32 (3): 202–8. doi:10.1159/000333072. PMID 22095040.
- ↑ Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y (2008). "Survival in Alzheimer disease: a multiethnic, population-based study of incident cases". Neurology. 71 (19): 1489–95. doi:10.1212/01.wnl.0000334278.11022.42. PMC 2843528. PMID 18981370.
- ↑ 69.0 69.1 69.2 69.3 69.4 Molloy S, McKeith IG, O'Brien JT, Burn DJ (2005). "The role of levodopa in the management of dementia with Lewy bodies". J Neurol Neurosurg Psychiatry. 76 (9): 1200–3. doi:10.1136/jnnp.2004.052332. PMC 1739807. PMID 16107351.
- ↑ O'Brien, John (2006). Dementia with Lewy Bodies and Parkinson's Disease Dementia. Taylor & Francis. Retrieved November 12 2014. Check date values in:
|accessdate=(help) - ↑ 71.0 71.1 Barber R, Panikkar A, McKeith IG (2001). "Dementia with Lewy bodies: diagnosis and management". Int J Geriatr Psychiatry. 16 Suppl 1: S12–8. PMID 11748785.
- ↑ 72.0 72.1 72.2 72.3 72.4 72.5 72.6 Shea C, MacKnight C, Rockwood K (1998). [http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?
dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9785144 "Donepezil for treatment of dementia with Lewy bodies: a case series of nine patients"] Check
|url=value (help). Int Psychogeriatr. 10 (3): 229–38. PMID 9785144. line feed character in|url=at position 54 (help) - ↑ 73.0 73.1 73.2 73.3 Emre M, Tsolaki M, Bonuccelli U, Destée A, Tolosa E, Kutzelnigg A; et al. (2010). "Memantine for patients with Parkinson's disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial". Lancet Neurol. 9 (10): 969–77. doi:10.1016/S1474-4422(10)70194-0. PMID 20729148.
- ↑ Minett TS, Thomas A, Wilkinson LM, Daniel SL, Sanders J, Richardson J; et al. (2003). "What happens when donepezil is suddenly withdrawn? An open label trial in dementia with Lewy bodies and Parkinson's disease with dementia". Int J Geriatr Psychiatry. 18 (11): 988–93. doi:10.1002/gps.995. PMID 14618549.
- ↑ Grace J, Daniel S, Stevens T, Shankar KK, Walker Z, Byrne EJ; et al. (2001). "Long-Term use of rivastigmine in patients with dementia with Lewy bodies: an open-label trial". Int Psychogeriatr. 13 (2): 199–205. PMID 11495394.
- ↑ Samuel W, Caligiuri M, Galasko D, Lacro J, Marini M, McClure FS; et al. (2000). "Better cognitive and psychopathologic response to donepezil in patients prospectively diagnosed as dementia with Lewy bodies: a preliminary study". Int J Geriatr Psychiatry. 15 (9): 794–802. PMID 10984725.
- ↑ Edwards KR, Hershey L, Wray L, Bednarczyk EM, Lichter D, Farlow M; et al. (2004). "Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 12-week interim analysis". Dement Geriatr Cogn Disord. 17 Suppl 1: 40–8. doi:10.1159/000074681. PMID 14676468.
- ↑ Boeve BF, Silber MH, Ferman TJ (2003). "Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients". Sleep Med. 4 (4): 281–4. PMID 14592300.
Template:Diseases of the nervous system Template:WH Template:WS
 KSF
KSF