Doripenem clinical pharmacology
 From Wikidoc - Reading time: 5 min
From Wikidoc - Reading time: 5 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Clinical Pharmacology[edit | edit source]
Doripenem is a carbapenem with in vitro antibacterial activity against aerobic and anaerobic Gram-positive and Gram-negative bacteria.
Mechanism of Action[edit | edit source]
Doripenem is an antibacterial drug. (see Microbiology)
Pharmacodynamics[edit | edit source]
Similar to other beta-lactam antimicrobial agents, the time that unbound plasma concentration of doripenem exceeds the MIC of the infecting organism has been shown to best correlate with efficacy in animal models of infection. However, the pharmacokinetic/pharmacodynamic relationship for doripenem has not been evaluated in patients.
In a randomized, positive- and placebo-controlled crossover QT study, 60 healthy subjects were administered DORIBAX® 500 mg IV every 8 hours × 4 doses and DORIBAX® 1g IV every 8 hours × 4 doses, placebo, and a single oral dose of positive control. At both the 500 mg and 1g DORIBAX® doses, no significant effect on QTc interval was detected at peak plasma concentration or at any other time.
Pharmacokinetics[edit | edit source]
Plasma Concentrations[edit | edit source]
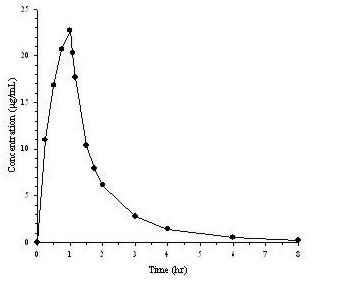
Mean plasma concentrations of doripenem following a single 1-hour intravenous infusion of a 500 mg dose of DORIBAX® to 24 healthy subjects are shown below in Figure 1. The mean (SD) plasma Cmax and AUC0–∞ values were 23.0 (6.6) µg/mL and 36.3 (8.8) µg∙hr/mL, respectively.
Figure 1. Average Doripenem Plasma Concentrations Versus Time Following a Single 1-Hour Intravenous Infusion of DORIBAX® 500 mg in Healthy Subjects (N=24)
 |
The pharmacokinetics of doripenem (Cmax and AUC) are linear over a dose range of 500 mg to 1g when intravenously infused over 1 hour. There is no accumulation of doripenem following multiple intravenous infusions of either 500 mg or 1g administered every 8 hours for 7 to 10 days in subjects with normal renal function. Distribution
The average binding of doripenem to plasma proteins is approximately 8.1% and is independent of plasma drug concentrations. The median (range) volume of distribution at steady state in healthy subjects is 16.8 L (8.09–55.5 L), similar to extracellular fluid volume (18.2 L).
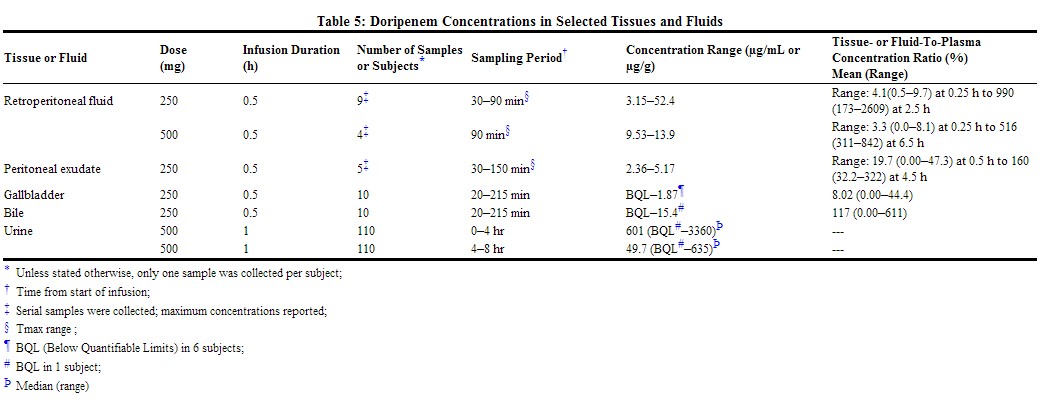
Doripenem penetrates into several body fluids and tissues, including those at the site of infection for the approved indications. Doripenem concentrations in peritoneal and retroperitoneal fluid either match or exceed those required to inhibit most susceptible bacteria; however, the clinical relevance of this finding has not been established. Concentrations achieved in selected tissues and fluids following administration of DORIBAX® are shown in Table 5:
 |
Metabolism[edit | edit source]
Metabolism of doripenem to a microbiologically inactive ring-opened metabolite (doripenem-M1) occurs primarily via dehydropeptidase-I. The mean (SD) plasma doripenem-M1-to-doripenem AUC ratio following single 500 mg and 1 g doses in healthy subjects is 18% (7.2%).
In pooled human liver microsomes, no in vitro metabolism of doripenem could be detected, indicating that doripenem is not a substrate for hepatic CYP450 enzymes.
Excretion[edit | edit source]
Doripenem is primarily eliminated unchanged by the kidneys. The mean plasma terminal elimination half-life of doripenem in healthy non-elderly adults is approximately 1 hour and mean (SD) plasma clearance is 15.9 (5.3) L/hour. Mean (SD) renal clearance is 10.3 (3.5) L/hour. The magnitude of this value, coupled with the significant decrease in the elimination of doripenem with concomitant probenecid administration, suggests that doripenem undergoes both glomerular filtration and active tubular secretion. In healthy adults given a single 500 mg dose of DORIBAX®, a mean of 71% and 15% of the dose was recovered in urine as unchanged drug and the ring-opened metabolite, respectively, within 48 hours. Following the administration of a single 500 mg dose of radiolabeled doripenem to healthy adults, less than 1% of the total radioactivity was recovered in feces after one week.
Special Populations[edit | edit source]
Patients with Renal Impairment[edit | edit source]
Following a single 500 mg dose of DORIBAX®, the mean AUC of doripenem in subjects with mild (CrCl 50–79 mL/min), moderate (CrCl 31–50 mL/min), and severe renal impairment (CrCl ≤30 mL/min) was 1.6-, 2.8-, and 5.1-times that of age-matched healthy subjects with normal renal function (CrCl ≥80 mL/min), respectively. Dosage adjustment is necessary in patients with moderate and severe renal impairment. [see Dosage and Administration (2.2)]
A single 500 mg dose of DORIBAX® was administered to subjects with end stage renal disease (ESRD) either one hour prior to or one hour after hemodialysis (HD). The mean doripenem AUC following the post-HD infusion was 7.8-times that of healthy subjects with normal renal function. The mean total recovery of doripenem and doripenem-M1 in the dialysate following a 4-hour HD session was 231 mg and 28 mg, respectively, or a total of 259 mg (52% of the dose). There is insufficient information to make dose adjustment recommendations in patients on hemodialysis.
Patients with Hepatic Impairment[edit | edit source]
The pharmacokinetics of doripenem in patients with hepatic impairment have not been established. As doripenem does not appear to undergo hepatic metabolism, the pharmacokinetics of doripenem are not expected to be affected by hepatic impairment.
Geriatric Patients[edit | edit source]
The impact of age on the pharmacokinetics of doripenem was evaluated in healthy male (n=6) and female (n=6) subjects ≥ 66 years of age. Mean doripenem AUC0–∞ was 49% higher in elderly adults relative to non-elderly adults. This difference in exposure was mainly attributed to age-related changes in creatinine clearance. No dosage adjustment is recommended for elderly patients with normal (for their age) renal function.
Gender[edit | edit source]
The effect of gender on the pharmacokinetics of doripenem was evaluated in healthy male (n=12) and female (n=12) subjects. Doripenem Cmax and AUC were similar between males and females. No dose adjustment is recommended based on gender.
Race[edit | edit source]
The effect of race on doripenem pharmacokinetics was examined using a population pharmacokinetic analysis of data from phase 1 and 2 studies. No significant difference in mean doripenem clearance was observed across race groups and therefore, no dosage adjustment is recommended based on race.
Drug Interactions[edit | edit source]
Administration of DORIBAX® 500 mg every 8 hours × 4 doses to 23 healthy male subjects receiving valproic acid 500 mg every 12 hours for 7 days decreased the mean Cmax of valproic acid by 44.5% (from 86.1 mcg/mL to 47.8 mcg/mL) and the mean Cmin by 77.7% (from 55.7 mcg/mL to 12.4 mcg/mL) compared to administration of valproic acid alone. The mean AUC0–tau of valproic acid also decreased by 63%. Conversely, the Cmax of the VPA-g metabolite was increased by 62.6% (from 5.19 mcg/mL to 8.44 mcg/mL) and the mean AUC0–tau of VPA-g was increased by 50%. The pharmacokinetics of doripenem were unaffected by the co-administration of valproic acid.(see Warnings and Precautions and Drug Interactions )
Probenecid interferes with the active tubular secretion of doripenem, resulting in increased plasma concentrations. Probenecid increased doripenem AUC by 75% and prolonged the plasma elimination half-life by 53%. (see also Drug Interactions )
In vitro studies in human liver microsomes and hepatocytes indicate that doripenem does not inhibit the major cytochrome P450 isoenzymes (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, and CYP4A11). Therefore, DORIBAX® is not expected to inhibit the clearance of drugs that are metabolized by these metabolic pathways in a clinically relevant manner.
DORIBAX® is also not expected to have CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP3A4/5, or UGT1A1 enzyme-inducing properties based on in vitro studies in cultured human hepatocytes.[1]
References[edit | edit source]
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022106s014lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.
 KSF
KSF