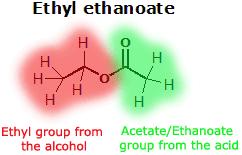
Ester
 From Wikidoc - Reading time: 6 min
From Wikidoc - Reading time: 6 min
Editor-In-Chief: Henry A. Hoff
Overview[edit | edit source]
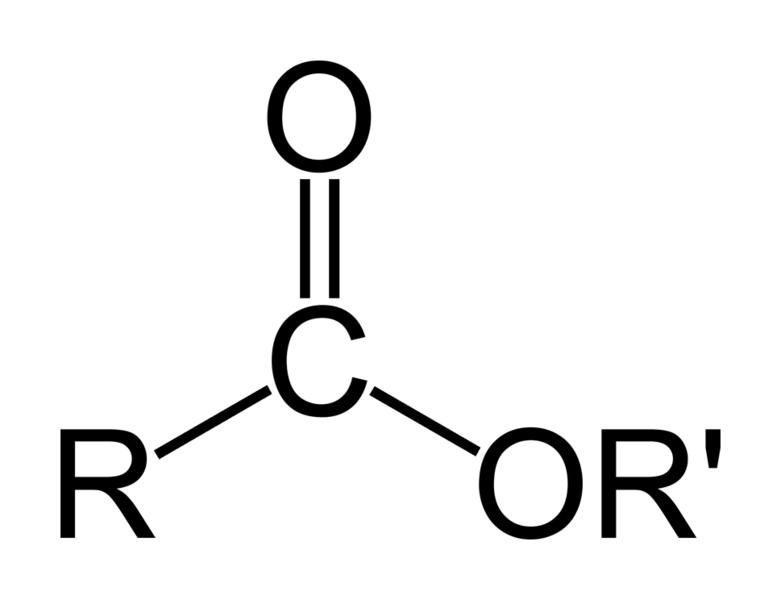
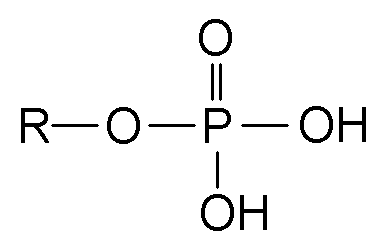
Esters are a class of chemical compounds and functional groups. Esters consist of an inorganic or organic acid in which at least one -OH (hydroxy) group is replaced by an -O-alkyl (alkoxy) group. The most common type of esters are carboxylic acid esters (R1-C(=O)-O-R2), other esters include phosphoric acid, sulfuric acid, nitric acid, and boric acid esters. Volatile esters often have a smell and are found in perfumes, essential oils, and pheromones and give many fruits their scent. Ethyl acetate and methyl acetate are important solvents, fatty acid esters form fat and lipids, and polyesters are important plastics. Cyclic esters are called lactones. The name "ester" is derived from the German Essig-Äther (literally:vinegar ether), an old name for ethyl acetate. Esters can be synthesized in a condensation reaction between an acid and an alcohol in a reaction known as esterification.
An ester is an often fragrant organic or partially organic compound formed by the reaction between an acid (including amino acids) and an alcohol (alkyl, R) or aromatic alcohol (aryl, R') (including a more basic amino acid) with the elimination of water. For examples,
acetic acid + an alcohol <=> acetic ester + water,
CH3COOH + ROH <=> CH3COOR + H2O,
or
CH3COO- + H+ + R+ + OH- <=> CH3COOR + H2O;
with one amino acid acting as a base:
formic acid + L-methionine <=> N-formyl-L-methionine (an amino acid) + H2O,
or
two amino acids:
Cys + Gly <=> Cys-Gly + H2O,
forming a dipeptide. A reaction between an inorganic hydroxide (e.g. sodium hydroxide) and an organic acid (e.g. acetic acid) produces a salt of acetic acid (sodium acetate). A compound is an ester when the hydroxide donor is organic and a salt when the hydroxide donor is inorganic. Hence, a carbonate can be thought of as a salt or an ester of carbonic acid.
Nomenclature[edit | edit source]
An ester is named according to the two parts that make it up: the part from the alcohol and the part from the acid (in that order), for example ethyl sulfuric acid ester.
Since most esters are derived from carboxylic acids, a specific nomenclature is used for them. For esters derived from the simplest carboxylic acids, the traditional name for the acid constituent is generally retained, e.g. formate, acetate, propionate, butyrate.[1] For esters from more complex carboxylic acids, the systematic name for the acid is used, followed by the suffix -oate. For example, methyl formate is the ester of methanol and methanoic acid (formic acid): the simplest ester. It could also be called methyl methanoate.[2]
Esters of aromatic acids are also encountered, including benzoates such as methyl benzoate, and phthalates, with substitution allowed in the name.
The chemical formulas of esters are typically in the format of R-COO-R', in which the alkyl group (R') is mentioned first, and the carboxylate group (R) is mentioned last.[3] For example the ester: butyl ethanoate - derived from butanol (C4H9OH) and ethanoic acid (CH3COOH) would have the formula: CH3COOC4H9. Sometimes the formula may be 'broken up' to show the structure, in this case: CH3COO[CH2]3CH3.
Oligoesters[edit | edit source]
The acetic ester, N-formyl-L-methionine, and the dipeptide examples above are each monoesters.
The term oligoester refers to any ester polymer containing a small number of component esters. As an example, chemically, fats are generally diesters of glycerol and fatty acids. Most of the mass of a fat/triester is in the 3 fatty acids.
Tetraesters can be found as part of membrane-spanning lipids in bacteria from the order Thermotogales.[4]
Pentaesters have been used as indicators[5] or in isotopic labelling[6] compounds.
Hexaesters such as calix[6]arene have been used in optodes as sensing devices for optical determination of potassium ion concentration in pH-buffer solutions.[7]
Heptaesters have been found in Euphorbia species.[8]
Octaesters can be inclusions of ester moieties within cavitand cavities.[9]
The number of esters can be up to ten as in oligo-(R)-3-hydroxybutyrate[10].
Physical properties[edit | edit source]
Esters participate in hydrogen bonds as hydrogen-bond acceptors, but cannot act as hydrogen-bond donors, unlike their parent alcohols. This ability to participate in hydrogen bonding makes them more water-soluble than their parent hydrocarbons. However, the limitations on their hydrogen bonding also make them more hydrophobic than either their parent alcohols or parent acids. Their lack of hydrogen-bond-donating ability means that ester molecules cannot hydrogen-bond to each other, which makes esters generally more volatile than a carboxylic acid of similar molecular weight. This property makes them very useful in organic analytical chemistry: unknown organic acids with low volatility can often be esterified into a volatile ester, which can then be analyzed using gas chromatography, gas liquid chromatography, or mass spectrometry. Many esters have distinctive odors, which has led to their use as artificial flavorings and fragrances. For example:
Ester synthesis[edit | edit source]
"Esterification" (condensation of an alcohol and an acid) is not the only way to synthesize an ester. Esters can be prepared in the laboratory in a number of other ways:
- by transesterifications between other esters
- by Dieckmann condensation or Claisen condensation of esters carrying acidic α-protons
- by Favorskii rearrangement of α-haloketones in presence of base
- by nucleophilic displacement of alkyl halides with carboxylic acid salts
- by Baeyer-Villiger oxidation of ketones with peroxides
- by Pinner reaction of nitriles with an alcohol
Ester reactions[edit | edit source]
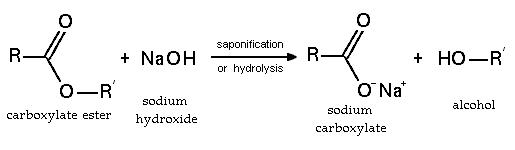
Esters react in a number of ways:
- Esters may undergo hydrolysis - the breakdown of an ester by water. This process can be catalyzed both by acids and bases. The base-catalyzed process is called saponification. The hydrolysis yields an alcohol and a carboxylic acid or its carboxylate salt.
- Esters also react if heated with primary or secondary amines, producing amides.
- Phenyl esters react to hydroxyarylketones in the Fries rearrangement.
- Di-esters such as diethyl malonate react as nucleophile with alkyl halides in the malonic ester synthesis after deprotonation.
- Specific esters are functionalized with an α-hydroxyl group in the Chan rearrangement
- Esters are converted to isocyanates through intermediate hydroxamic acids in the Lossen rearrangement.
- Esters with β-hydrogen atoms can be converted to alkenes in ester pyrolysis
External links[edit | edit source]
- An introduction to esters
- Molecule of the month: Ethyl acetate and other esters
- Making an Ester A simple guide to naming and making esters, as well as the chemistry behind it.
References[edit | edit source]
- ↑ IUPAC parent groups using traditional names
- ↑ IUPAC naming of esters
- ↑ http://www.acdlabs.com/iupac/nomenclature/93/r93_511.htm
- ↑ Damsté JS, Rijpstra WI, Hopmans EC, Schouten S, Balk M, Stams AJ (2007). "Structural characterization of diabolic acid-based tetraester, tetraether and mixed ether/ester, membrane-spanning lipids of bacteria from the order Thermotogales". Arch Microbiol. 188 (6): 629–41. doi:10.1007/s00203-007-0284-z. PMID 17643227. Unknown parameter
|month=ignored (help) - ↑ Najafi A, Fawcett HD, Hutchison N (1986). "Sarcoplasmic reticulum interacts with the Ca(2+) indicator precursor fura-2-am". Biochem Biophys Res Commun. 138 (3): 1153–62. doi:10.1016/S0006-291X(86)80403-X. PMID 3755905. Unknown parameter
|month=ignored (help) - ↑ Highsmith S, Bloebaum P, Snowdowne KW (1986). "Comparison of two methods of labeling proteins with 111In". Int J Rad Appl Instrum B. 13 (4): 345–6. PMID 3539883.
- ↑ Chan WH, Lee AW, Kwong DW, Tam WL, Wang KM (1996). "Potassium ion-selective optodes based on the calix[6]arene hexaester and application in human serum assay". Analyst. 121 (4): 531–4. doi:10.1039/an9962100531. PMID 8633794. Unknown parameter
|month=ignored (help) - ↑ Evanics F, Hohmann J, Rédei D, Vasas A, Günther G, Dombi G (2001). "[New diterpene polyesters isolated from Hungarian Euphorbia species] [Article in Hungarian]". Acta Pharm Hung. 71 (3): 289–92. PMID 11961895. Unknown parameter
|month=ignored (help) - ↑ Dueno EE, Bisht KS (2004). "Intramolecular inclusion in novel octaester cavitands". Chem Commun (Camb). (8): 954–5. doi:10.1039/b316498e. PMID 15069491. Unknown parameter
|month=ignored (help) - ↑ Xian M, Fuerst MM, Shabalin Y, Reusch RN (2007). "Sorting signal of Escherichia coli OmpA is modified by oligo-(R)-3-hydroxybutyrate". Biochim Biophys Acta. 1768 (11): 2660–6. doi:10.1016/j.bbamem.2007.06.019. PMID 17659252. Unknown parameter
|month=ignored (help)
ar:إستر
bg:Естер
ca:Èster
cs:Estery
da:Ester
de:Ester
et:Estrid
eo:Estero
ko:에스터
hr:Esteri
id:Ester
it:Esteri
he:אסטר
la:Ester
lv:Esteri
lt:Esteriai
mk:Естер
nl:Ester (chemie)
no:Ester
nn:Ester
sk:Ester (zlúčenina)
sl:Ester
sr:Estar
sh:Estri
su:Éster
fi:Esteri
sv:Estrar
uk:Естери
 KSF
KSF