Ether
 From Wikidoc - Reading time: 6 min
From Wikidoc - Reading time: 6 min
|
WikiDoc Resources for Ether |
|
Articles |
|---|
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ether at Clinical Trials.gov Clinical Trials on Ether at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ether
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Ether Risk calculators and risk factors for Ether
|
|
Healthcare Provider Resources |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview[edit | edit source]
Ether is the general name for a class of chemical compounds which contain an ether group — an oxygen atom connected to two (substituted) alkyl or aryl groups — of general formula R – O–R'.[1] A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether" (ethoxyethane, CH3-CH2-O-CH2-CH3).
Physical properties[edit | edit source]
Ether molecules cannot form hydrogen bonds among each other, resulting in a relatively low boiling point comparable to that of the analogous alcohols. However, the differences in the boiling points of the ethers and their isometric alcohols become smaller as the carbon chains become longer, as the hydrophobic nature of the carbon chain becomes more predominant over the presence of hydrogen bonding.
Ethers are slightly polar as the C - O - C bond angle in the functional group is about 110 degrees, and the C - O dipole does not cancel out. Ethers are more polar than alkenes but not as polar as alcohols, esters or amides of comparable structure. However, the presence of two lone pairs of electrons on the oxygen atoms makes hydrogen bonding with water molecules possible, causing the solubility of alcohols (for instance, butan-1-ol) and ethers (ethoxyethane) to be quite dissimilar.
Cyclic ethers such as tetrahydrofuran and 1,4-dioxane are totally miscible in water because of the more exposed oxygen atom for hydrogen bonding as compared to aliphatic ethers.
Ethers can act as Lewis bases. For instance, diethyl ether forms a complex with boron compounds, such as boron trifluoride diethyl etherate (BF3.OEt2). Ethers also coordinate to magnesium in Grignard reagents (RMgBr).
Nomenclature[edit | edit source]
In the IUPAC nomenclature system, ethers are named using the general formula "alkoxyalkane", for example CH3-CH2-O-CH3 is methoxyethane. If the ether is part of a more complex molecule, it is described as an alkoxy substituent, so -OCH3 would be considered a "methoxy-" group. The simpler alkyl radical is written in front, so CH3-O-CH2CH3 would be given as methoxy(CH3)ethane(CH2CH3). The nomenclature of describing the two alkyl groups and appending "ether", e.g. "ethyl methyl ether" in the example above, is a trivial usage.
Similar structures[edit | edit source]

Ethers are not to be confused with the following classes of compounds with the same general structure R-O-R.
- Aromatic compounds like furan where the oxygen is part of the aromatic system.
- Compounds where one of the carbon atoms next to the oxygen is connected to oxygen, nitrogen, or sulfur:
- Esters R-C(=O)-O-R
- Acetals R-CH(-O-R)-O-R
- Aminals R-CH(-NH-R)-O-R
- Anhydrides R-C(=O)-O-C(=O)-R
Primary, secondary, and tertiary ethers[edit | edit source]
The terms "primary ether", "secondary ether", and "tertiary ether" are occasionally used and refer to the carbon atom next to the ether oxygen. In a primary ether this carbon is connected to only one other carbon as in diethyl ether CH3-CH2-O-CH2-CH3. An example of a secondary ether is diisopropyl ether (CH3)2CH-O-CH(CH3)2 and that of a tertiary ether is di-tert-butyl ether (CH3)3C-O-C(CH3)3.
Dimethyl ether, a primary, a secondary, and a tertiary ether.
Polyethers[edit | edit source]
Polyethers are compounds with more than one ether group. While the term generally refers to polymers like polyethylene glycol and polypropylene glycol, low molecular compounds such as the crown ethers may sometimes be included.
Organic reactions[edit | edit source]
Synthesis[edit | edit source]
Ethers can be prepared in the laboratory in several different ways.
- R-OH + R-OH → R-O-R + H2O
- This direct reaction requires drastic conditions (heating to 140 degrees Celsius and an acid catalyst, usually concentrated sulphuric acid). Effective for making symmetrical ethers, but not as useful for synthesising asymmetrical ethers because the reaction will yield a mixture of ethers, making it usually not applicable:
- 3R-OH + 3R'-OH → R-O-R + R'-O-R + R'-O-R' + 3H2O
- Conditions must also be controlled to avoid overheating to 170 degrees which will cause intramolecular dehydration,a reaction that yields alkenes. In addition, the alcohol must be in excess.
- R-CH2-CH2(OH) → R-CH=CH2 + H2O
- Such conditions can destroy the delicate structures of some functional groups. There exist several milder methods to produce ethers.
- R-O- + R-X → R-O-R + X-
- This reaction is called the Williamson ether synthesis. It involves treatment of a parent alcohol with a strong base to form the alkoxide anion followed by addition of an appropriate aliphatic compound bearing a suitable leaving group (R-X). Suitable leaving groups (X) include iodide, bromide, or sulfonates. This method does not work if R is aromatic like in bromobenzene (Br-C6H5), however, if the leaving group is separated by at least one carbon from the benzene, the reaction should procede (as in Br-CH2-C6H5). Likewise, this method only gives the best yields for primary carbons, as secondary and tertiary carbons will undergo E2 elimination on exposure to the basic alkoxide anion used in the reaction due to steric hindrance from the large alkyl groups. Aryl ethers can be prepared in the Ullmann condensation.
- Nucleophilic Displacement of Alkyl halides by phenoxides
- The R-X cannot be used to react with the alcohol. However, phenols can be used to replace the alcohol, while maintaining the alkyl halide. Since phenols are acidic, they readily react with a strong base like sodium hydroxide to form phenoxide ions. The phenoxide ion will then substitute the -X group in the alkyl halide, forming an ether with an aryl group attached to it in a reaction with an SN2 mechanism.
- HO-C6H5 + OH- → O--C6H5
- O--C6H5 + R-X → R-O-C6H5
- Electrophilic addition of alcohols to alkenes.
- R2C=CR2 + R-OH → R2CH-C(-O-R)-R2
- Acid catalysis is required for this reaction. Often, Mercury trifluoroacetate (Hg(OCOCF3)2 is used as a catalyst for the reaction, creating an ether with Markovnikov regiochemistry. Tetrahydropyranyl ethers are used as protective groups for alcohols.
Cyclic ethers which are also known as epoxides can be prepared:
- By the oxidation of alkenes with a peroxyacid such as m-CPBA.
- By the base intramolecular nuclephilic substitution of a halohydrin.
Reactions[edit | edit source]
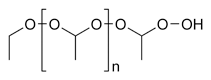
Ethers in general are of very low chemical reactivity. Organic reactions are:
- Ethers are hydrolyzed only under drastic conditions like heating with boron tribromide or boiling in hydrobromic acid. Lower mineral acids containing a halogen, such as hydrochloric acid will cleave ethers, but very slowly. Hydrobromic acid and hydroiodic acid are the only two that do so at an appreciable rate. Certain aryl ethers can be cleaved by aluminium chloride.
- Epoxides, or cyclic ethers in three-membered rings, are highly susceptible to nucleophilic attack and are reactive in this fashion.
- Peroxide formation.
- Primary and secondary ethers with a CH group next to the ether oxygen easily form highly explosive organic peroxides (e.g. diethyl ether peroxide) in the presence of oxygen, light, and metal and aldehyde impurities. For this reason ethers like diethyl ether and THF are usually avoided as solvents in industrial processes.
Important ethers[edit | edit source]
| Ethylene oxide | The smallest cyclic ether. | |
| Dimethyl ether | An aerosol spray propellant. | |
| Diethyl ether | A common low boiling solvent (b.p. 34.6°C). | |
|
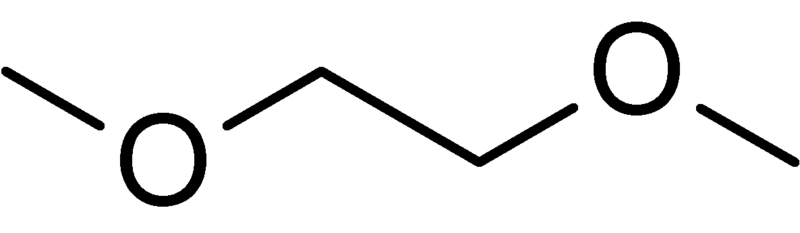
Dimethoxyethane (DME) | A high boiling solvent (b.p. 85°C): |

|
Dioxane | A cyclic ether and high boiling solvent (b.p. 101.1°C). |
|
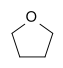
Tetrahydrofuran (THF) | A cyclic ether, one of the most polar simple ethers that is used as a solvent. |
|
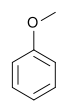
Anisole (methoxybenzene) | An aryl ether and a major constituent of the essential oil of anise seed. |
|
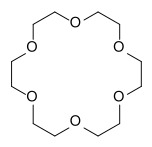
Crown ethers | Cyclic polyethers that are used as phase transfer catalysts. |
| Polyethylene glycol (PEG) | A linear polyether, e.g. used in cosmetics: |
See also[edit | edit source]
- Functional group
- Methoxy
- Petroleum ether, not an ether but a low boiling alkane mixture.
- Thioether, analogs of ethers with the oxygen replaced by sulfur.
- Luminiferous ether
References[edit | edit source]
External links[edit | edit source]
- ILPI page about ethers.
- An Account of the Extraordinary Medicinal Fluid, called Aether, by M. Turner, circa 1788, from Project Gutenberg
- The Ether Monument at Boston Public Gardens
ar:إيثر bg:Етер ca:Èter da:Æter (kemi) de:Ether et:Eetrid eo:Etero ko:에테르 (화학) it:Eteri he:אתר (כימיה) la:Aether (chemica) lv:Ēteri hu:Éter (kémia) mk:Етер nl:Ether (chemie) no:Eter nn:Eterar sl:Eter sr:Етар (хемија) fi:Eetteri sv:Etrar uk:Етери
 KSF
KSF