Gallamine
 From Wikidoc - Reading time: 3 min
From Wikidoc - Reading time: 3 min
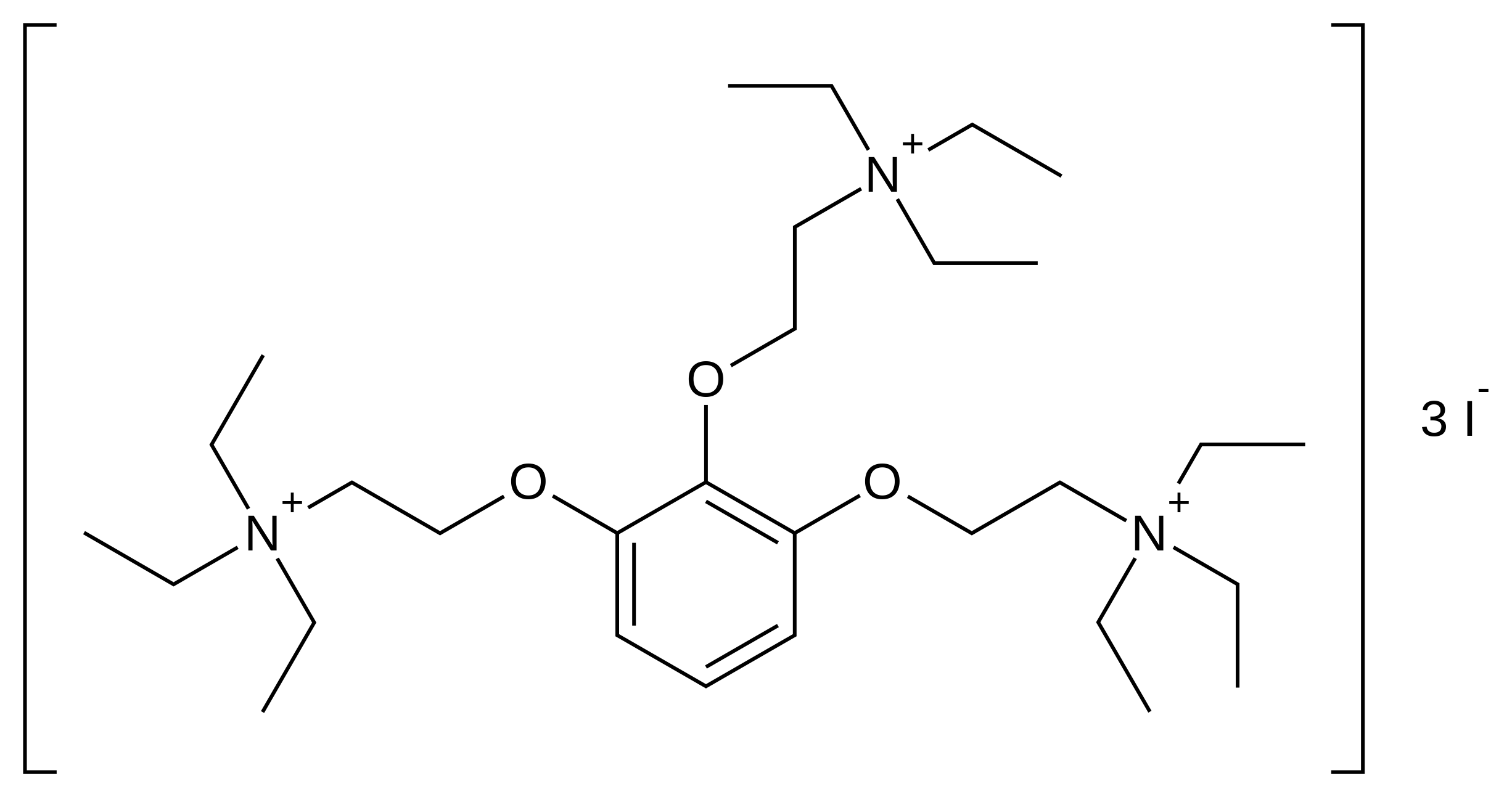 | |
| Clinical data | |
|---|---|
| Trade names | Flaxedil |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C30H60N3O3+3 · 3 I− (gallamine triethiodide) C24H45N3O3 (gallamine) |
| Molar mass | 891.529 g/mol (gallamine triethiodide) 423.633 g/mol (gallamine) |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Gallamine |
|
Articles |
|---|
|
Most recent articles on Gallamine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Gallamine at Clinical Trials.gov Clinical Trials on Gallamine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Gallamine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Gallamine Discussion groups on Gallamine Directions to Hospitals Treating Gallamine Risk calculators and risk factors for Gallamine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Gallamine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview[edit | edit source]
Gallamine triethiodide (Flaxedil) is a non-depolarising muscle relaxant.[1] It acts by combining with the cholinergic receptor sites in muscle and competitively blocking the transmitter action of acetylcholine.[2] Gallamine triethiodide has a parasympatholytic effect on the cardiac vagus nerve which causes tachycardia[3][4] and occasionally hypertension. Very high doses cause histamine release.
Gallamine triethiodide is commonly used to stabilize muscle contractions during surgical procedures.
It was developed by Daniel Bovet in 1947.[5]
The pharmaceutical is no longer marketed in the United States, according to the FDA Orange Book.
References[edit | edit source]
- ↑ "Webster's Online Dictionary - Flaxedil". Retrieved 2008-12-15.
- ↑ "RxMed: Pharmaceutical Information - FLAXEDIL". Retrieved 2008-12-15.
- ↑ Morgenstern C, Splith G (October 1965). "[Studies on the causes of gallamine tachycardia and its antagonistic modification by beta adrenolytics]". Der Anaesthesist (in German). 14 (10): 298–301. PMID 4380161.
|access-date=requires|url=(help) - ↑ WALTS LF (1963). "Ventricular tachycardia with gallamine and cyclopropane anesthesia". Anesthesiology. 24: 119. doi:10.1097/00000542-196301000-00024. PMID 13998750. Retrieved 2014-09-20.
- ↑ Raghavendra T (July 2002). "Neuromuscular blocking drugs: discovery and development". J R Soc Med. 95 (7): 363–7. doi:10.1258/jrsm.95.7.363. PMC 1279945. PMID 12091515.
 KSF
KSF