Heterocyclic compound
 From Wikidoc - Reading time: 4 min
From Wikidoc - Reading time: 4 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview[edit | edit source]
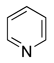
Heterocyclic compounds are organic compounds that contain a ring structure containing atoms in addition to carbon, such as sulfur, oxygen or nitrogen, as part of the ring. They may be either simple aromatic rings or non-aromatic rings. Some examples are pyridine (C5H5N), pyrimidine (C4H4N2) and dioxane (C4H8O2).
Note that compounds such as cyclopropane, an anaesthetic with explosive properties, and cyclohexane, a solvent, are not heterocyclic, they are merely cycloalkanes. The suffix '-cyclic' implies a ring structure, while 'hetero' refers to an atom other than carbon, as above. Many heterocyclic compounds, including some amines, are carcinogenic.
Heterocyclic chemistry is the chemistry branch dealing exclusively with synthesis, properties and applications of heterocycles especially vital to drug design.
3-membered rings[edit | edit source]
Heterocycles with three atoms in the ring are more reactive because of ring strain. Those containing one heteroatom are generally stable. Those with two heteroatoms are more likely to occur as reactive intermediates. Common 3-membered heterocycles are:
| heteroatom | saturated | unsaturated |
|---|---|---|
| Nitrogen | aziridine | |
| Oxygen | ethylene oxide (epoxides, oxiranes) | oxirene |
| Sulphur | thiirane (episulfides) |
4-membered rings[edit | edit source]
| heteroatom | saturated | unsaturated |
|---|---|---|
| Nitrogen | azetidine | |
| Oxygen | oxetane |
5-membered rings[edit | edit source]
With heterocycles containing five atoms, the unsaturated compounds are frequently more stable because of aromaticity.
| heteroatom | saturated | unsaturated |
|---|---|---|
| Nitrogen | dihydropyrrole (pyrroline) & tetrahydropyrrole (pyrrolidine) | pyrrole |
| Oxygen | dihydrofuran & tetrahydrofuran | furan |
| Sulphur | dihydrothiophene & tetrahydrothiophene | thiophene (thiole) |
| Arsenic | arsole |
With two heteroatoms:
- The azoles:
- Two S: Dithiolane
6-membered rings[edit | edit source]
| heteroatom | saturated | unsaturated |
|---|---|---|
| Nitrogen | piperidine | pyridine |
| Oxygen | tetrahydropyran | pyran |
| Sulphur | Thiane | Thiine aka thiapyrane |
With two heteroatoms:
- Two N: Pyridazine, Pyrimidine, and Pyrazine are the 1,2-, 1,3-, and 1,4-isomers, respectively.
- Two N: Piperazine
- One N and one O: Oxazines
- One N and one S: Thiazine
- Two S: Dithiane
- Two O: Dioxane
Heterocyclic amines and cancer[edit | edit source]
Some heterocyclic amines (HCAs) found in cooked meat are known carcinogens. Research has shown that cooking certain meats at high temperatures creates chemicals that are not present in uncooked meats. For example, heterocyclic amines are the carcinogenic chemicals formed from the cooking of muscle meats such as beef, pork, fowl, and fish. HCAs form when amino acids and creatine (a chemical found in muscles) react at high cooking temperatures. Researchers have identified 17 different HCAs resulting from the cooking of muscle meats that may pose human cancer risk.[1] NCI's Division of Cancer Epidemiology and Genetics found a link between individuals with stomach cancer and the consumption of cooked meat, and other studies for colorectal, pancreatic, and breast cancer is associated with high intakes of well-done, fried, or barbecued meats. Other sources of protein (milk, eggs, tofu, and organ meats such as liver) have very little or no HCA content naturally or when cooked.
References[edit | edit source]
- ↑ "Heterocyclic Amines in Cooked Meats". National Cancer Institute. 15 Sep 2004. Retrieved 2007-08-09.
External links[edit | edit source]
- Heterocyclic amines in cooked meat, US CDC
- List of known and probable carcinogens, American Cancer Society
- List of known carcinogens by the State of California, Proposition 65 (more comprehensive)
ar:حلقة غير متجانسة ca:Compost heterocíclic cs:Heterocyklické sloučeniny de:Heterocyclen it:Composto eterociclico he:תרכובת הטרוציקלית mk:Хетероциклично соединение nl:Heterocyclische verbinding sk:Heterocyklická zlúčenina fi:Heterosyklinen yhdiste sv:Heterocykliska föreningar uk:Гетероциклічні сполуки
 KSF
KSF