Kidney transplantation
 From Wikidoc - Reading time: 11 min
From Wikidoc - Reading time: 11 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Dildar Hussain, MBBS [2]
Overview[edit | edit source]
Kidney (renal) transplantation describes the process of transplanting a kidney in a patient with end-stage renal disease. Kidney transplantation is typically categorized into two groups, depending on the source of the recipient organ, deceased donor (previously known as cadaveric) and living-donor. Living-donor renal transplants can be further categorized based on whether there is a biological relationship between the donor and recipient into genetically related (living-related) or non-related (living-unrelated). The indication for kidney transplantation is end-stage renal disease (ESRD), regardless of the primary cause. This is defined as a drop in the glomerular filtration rate (GFR) to 20-25% of normal. Studies show that kidney transplantation is a life-extending procedure. The typical patient will live ten to fifteen years longer with a kidney transplant than if kept on dialysis.
History[edit | edit source]
- On June 17, 1950, in Chicago, Illinois, the first known kidney transplant was performed on Ruth Tucker, a 44-year-old woman with polycystic kidney disease. Even without immunosuppressive therapy, the development of effective anti-rejection drugs was years away. Tucker lived another 5 years before dying of an unrelated illness.
- In order to eliminate any problems of an immune reaction, a transplantation was done in Boston between identical twins.
- It was not until 1960 that the first kidney transplant in the United Kingdom occurred when Michael Woodruff performed one between identical twins in Edinburgh.
- Decreased donor transplantation could not be performed until the routine use of medications to prevent and treat acute rejection was introduced in 1964. Being as tissue typing was simple, the organ was relatively easy to remove and implant, live donors could be used without difficulty, and kidney dialysis was available from the 1940s in the event of failure, the kidney was and is the easiest organ to transplant. Tissue typing was essential to the success of kidney transplantation being as early attempts in the 1950s on sufferers from Bright's disease had been very unsuccessful. However, in 1954, Dr. Joseph E. Murray performed the world's first successful renal transplant between genetically non-identical patients leading him to win the Nobel Prize for Medicine in 1990.
- The major barrier to organ transplantation between genetically non-identical patients was recipient's immune system and its treatment of a transplanted kidney as a "non-self" and (immediately or chronically), reject it. Thus, it was crucial to have medications to suppress the immune system.
- This suppression of an individual's immune system places that individual at greater risk of:
- Infection
- Cancer (particularly skin cancer and lymphoma)
- Side effects of the medications.
- The basis for most immunosuppressive regimens begins with prednisone, which is a corticosteroid. Prednisone suppresses the immune system, however, its long-term use at high doses carries many side effects, including:
- Glucose intolerance
- Diabetes
- Weight gain
- Osteoporosis
- Muscle weakness
- Hypercholesterolemia
- Cataract formation
- Prednisone is usually not adequate on its own to prevent rejection of a transplanted kidney. Thus, the need for other, non-steroid, immunosuppressive agents for two reasons:
- To combine it with prednisone to prevent rejection
- To allow lower doses of prednisone, or steroid-free regimens
Indications[edit | edit source]
- The indication for kidney transplantation is end-stage renal disease (ESRD), regardless of the primary cause. This is defined as a drop in the glomerular filtration rate (GFR) to 20-25% of normal.
- Common diseases leading to ESRD include:
- Genetic causes include:
- Polycystic kidney disease
- Inborn errors of metabolism
- Autoimmune conditions including lupus and Goodpasture's syndrome
- Diabetes is the most common cause of kidney transplant, accounting for approximately 25% of those in the US.
- The majority of renal transplant recipients are on some form of dialysis – hemodialysis, peritoneal dialysis, or the similar process of hemofiltration – at the time of transplantation. However, individuals with chronic renal failure who have a living donor available often elect to undergo transplantation before dialysis is needed.
Contraindications[edit | edit source]
Contraindications include:
- Cardiac insufficiency
- Pulmonary insufficiency
- Hepatic disease
- Concurrent tobacco use and morbid obesity are also among the indicators putting a patient at a higher risk for surgical complications
- Recent cancer, active substance abuse, or failure to adhere to prescribed medical regimens may make someone ineligible for a transplant.
Sources of kidneys[edit | edit source]
Since medication to prevent rejection is very effective, donors need not be genetically similar to their recipient. Most donated kidneys come from deceased donors, with some coming from living donors. However, the utilization of living donors in the United States is on the rise. In the year 2006, 47% of donated kidneys were actually from living donors (Organ Procurement and Transplantation Network, 2007). It is important to note that this varies by country: for example, only 3% of transplanted kidneys during 2006 in Spain came from living donors (Organización Nacional de Transplantes (ONT), 2007).
Living donors[edit | edit source]
Potential donors are carefully evaluated on medical and psychological grounds. This ensures that the donor is fit for surgery and has no kidney disease whilst confirming that the donor is purely altruistic. Traditionally the donor procedure has been through an incision but live donation has increasingly proceeded by laproscopic surgery. This reduces pain and accelerates the return to work for the donor with minimal effect on the outcome of the kidney. Overall, recipients of kidneys from live donors do relatively well, in comparison to deceased donors. In 2004 the FDA approved the Cedars-Sinai High Dose IVIG therapy which eliminates the need for the living donor to be the same blood type (ABO-compatible) or even a tissue match. The therapy stops the recipient's immune system from rejecting the donated kidney.
Deceased Donors[edit | edit source]
Deceased donors can be divided in two groups:
- Brain-dead (BD) donors
- Donation after Cardiac Death (DCD) donors
Although brain-dead (or "heart-beating") donors are considered dead, the donor's heart continues to pump and maintain the circulation. This makes it possible for surgeons to start operating while the organs are still being perfused. During the operation, the aorta will be cannulated, after which the donor's blood will be replaced by an ice-cold storage solution, such as UW (Viaspan), HTK, or Perfadex. [Depending on which organs are transplanted, more than one solution may be used simultaneously.] Due to the temperature of the solution (and since large amounts of cold NaCl-solution are poured over the organs for a rapid cooling of the organs), the heart will stop pumping.
"Donation after Cardiac Death" donors are patients who do not meet the brain-dead criteria, but have no chance of recovery whatsoever. In this procedure, the treatment is abstained (mechanical ventilation is shut off). Usually, a certain amount of minutes after death has been pronounced, the patient is rushed to the operating theatre, where the organs are procured, after which the storage solution is flushed through the organs itself. Since the blood is no longer being circulated, coagulation must be prevented with relatively large amounts of anti-coagulation agents, such as heparin.
Kidneys from brain-dead donors are generally of a superior quality since they have not been exposed to warm ischemia (the time between the stopping and the kidney being cooled).
Compatibility[edit | edit source]
The donor and recipient generally have to be ABO blood group compatible, although some programs are experimenting with ABO-incompatible transplantation using increased immunosuppression and plasmapheresis. Also, they should ideally share as many HLA and "minor antigens" as possible. This decreases the risk of transplant rejection and the need for another transplant. The risk of rejection may be further reduced if the recipient is not already sensitized to potential donor HLA antigens and if immunosuppressant levels are kept in an appropriate range. In the United States, up to 17% of all deceased donor kidney transplants have no HLA mismatch. However, it is important to note that HLA matching is a relatively minor predictor of transplant outcomes. In fact, living non-related donors are now almost as common as living (genetically)-related donors. In 2004 the FDA approved the Cedars-Sinai High Dose IVIG protocol which eliminates the need for the donor to be the same blood type or even a good tissue match.
While race has typically been used as criteria to judge compatibility, the validity of such criteria has presently come into question. Recently, certain genes have been linked to kidney-transplant failure[1]. Studies have shown that a specific gene known as APOL1 is associated with many kidney diseases, suggesting that they could cause failure in donated kidneys. While race and ethnicity are a part of the KDPI index (a scoring system used to match a kidney donor and recipient) and the APOL1 gene is found almost exclusively in people of African-decent, only 13% of this population has high-risk APOL1 variants that could cause kidney problems. Therefore, these recent studies have been showing that race might not be a key factor to consider.
Procedure[edit | edit source]
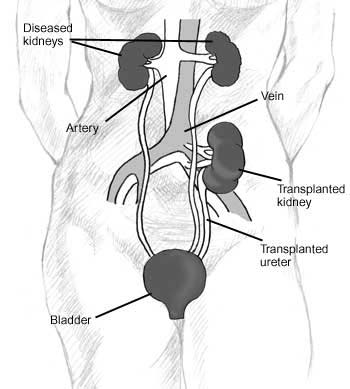
Since in most cases the barely functioning existing kidneys are not removed because this has been shown to increase the rates of surgical morbidities, the kidney is usually placed in a location different from the original kidney (often in the iliac fossa), and as a result it is often necessary to use a different blood supply:
- The renal artery of the kidney, previously branching from the abdominal aorta in the donor, is often connected to the external iliac artery in the recipient.
- The renal vein of the new kidney, previously draining to the inferior vena cava in the donor, is often connected to the external iliac vein in the recipient.
Kidney-pancreas transplant[edit | edit source]
Template:Seealso Occasionally, the kidney is transplanted together with the pancreas. This is done in patients with diabetes mellitus type I, in whom the diabetes is due to the destruction of the beta cells of the pancreas and in whom diabetes has caused renal failure (diabetic nephropathy). This is almost always a deceased donor transplant. Only a few living donor (partial) pancreas transplants have been done. For individuals with diabetes and renal failure, the advantages of earlier transplant from a living donor are approximately equal to the risks of continued dialysis until a combined kidney and pancreas are available from a deceased donor.
These procedures are commonly abbreviated as follows:
- "SKP transplant", for "simultaneous kidney-pancreas transplant"
- "PAK transplant", for "pancreas after kidney transplant"
(By contrast, "PTA" refers to "Pancreas transplant alone".)
The pancreas can come from a deceased donor as well as a living one. A patient can either receive a living kidney followed by a donor pancreas at a later date (PAK, or pancreas-after-kidney) or a combined kidney-pancreas from a donor (SKP, simultaneous kidney-pancreas.)
Transplanting just the islet cells from the pancreas is still in the experimental stage but shows promise. This involves taking a deceased donor pancreas, breaking it down, and extracting the islet cells that make insulin. The cells are then injected through a catheter into the recipient and they generally lodge in the liver. The recipient still needs to take immunosuppressants to avoid rejection, but no surgery is required. Most people need two or three such injections, and many are not completely insulin-free.
Post operation[edit | edit source]
The transplant surgery lasts about three hours. The donor kidney will be placed in the lower abdomen and its blood vessels connected to the recipient's blood vessels. When this is complete, blood will be allowed to flow through the kidney again, so the time for ischemia is minimized. In most cases, the kidney will soon start producing urine. Since urine is sterile, this has no effect on the surgery. The final step is connecting the ureter from the donor kidney to the bladder.
Depending on its quality, the new kidney usually begins functioning immediately. Living donor kidneys normally require 3-5 days to reach normal functioning levels, while cadaveric donations stretch that interval to 7-15 days. Hospital stay is typically for four to seven days. If complications arise, additional medicines may be administered to help the kidney produce urine.
Medicines are used to suppress the immune system from rejecting the donor kidney. These medicines must be taken for the rest of the patient's life.
The most common medication regimen today is:
Some patients may instead take:
Cyclosporine, is considered a breakthrough immuno-suppressive when first discovered in the 1980's, ironically causes nephrotoxicity and can result in iatrogenic damage to the newly transplanted kidney. Blood levels must be monitored closely and if the patient seems to have a declining renal function, a biopsy may be necessary to determine if this is due to rejection or cyclosporine intoxication.
Acute rejection occurs in 10% to 25% of people after transplant during the first sixty days. Rejection does not necessarily mean loss of the organ, but may require additional treatment.[3]
Complications[edit | edit source]
Complications after a transplant may include:
- Transplant rejection (hyperacute, acute or chronic)
- Infections and sepsis due to the immunosuppressant drugs that are required to decrease risk of rejection
- Post-transplant lymphoproliferative disorder (a form of lymphoma due to the immune suppressants)
- Imbalances in electrolytes including calcium and phosphate which can lead to bone problems amongst other things
- Other side effects of medications including gastrointestinal inflammation and ulceration of the stomach and esophagus, hirsutism, hair loss, obesity, acne, diabetes mellitus (type 2), hypercholesterolemia, and others
- The average lifetime for a donor kidney is ten to fifteen years. When a transplant fails a patient may opt for a second transplant, and may have to return to dialysis for some intermediary time
Recent studies suggest a link between skin cancer development and kidney transplants[2]. During a kidney transplant, the recipient is left open to cancer-causing viruses like HPV due to the immunosuppressants that they are given. These viruses can spread and cause cancer unchecked.
Prognosis[edit | edit source]
- Studies show that kidney transplantation is a life-extending procedure.[3]
- The typical patient will live ten to fifteen years longer with a kidney transplant than if kept on dialysis.[citation needed]
- The years of life gained is greater for younger patients, but even 75-year-old recipients (the oldest group for which there is data) gain an average four more years' life.
- People generally have more energy, a less restricted diet, and fewer complications with a kidney transplant than if they stay on conventional dialysis.
- Some studies seem to suggest that the longer a patient is on dialysis before the transplant, the less time the kidney will last. It is not clear why this occurs, but it underscores the need for rapid referral to a transplant program. Ideally, a kidney transplant should be pre-emptive, i.e. take place before the patient starts on dialysis.
- At least three professional athletes have made a comeback to their sport after receiving a transplant: NBA players Sean Elliott and Alonzo Mourning; and New Zealand rugby union legend Jonah Lomu as well as the German-Croatian Soccer Player Ivan Klasnic.
Kidney transplant requirements[edit | edit source]
- Kidney transplant requirements vary from program to program and country to country. Many programs place limits on age (e.g. the person must be less than 69 years old when put on the waiting list) and require that one must be in good health (aside from the kidney disease).
- Transplant exclusion criteria: Significant cardiovascular disease, incurable terminal infectious diseases and cancer.
- HIV was at one point considered to be a complete contraindication to transplantation. There was fear that immunosuppressing someone without a depleted immune system would result in the progression of the disease. However, current research does not bear out this fear; in fact, there are findings that immunosuppressive drugs and antiretrovirals may work synergistically to help both HIV viral loads/CD4 cell counts and prevent active rejection.
Kidney transplant statistics[edit | edit source]
| Country | Year | Cadaveric donor | Living donor | Total transplants |
|---|---|---|---|---|
| Canada[4] | 2000 | 724 | 388 | 1112 |
| France[5] | 2003 | 1991 | 136 | 2127 |
| Italy[5] | 2003 | 1489 | 135 | 1624 |
| Spain[5] | 2003 | 1991 | 60 | 2051 |
| United Kingdom[5] | 2003 | 1297 | 439 | 1736 |
| United States[6] | 2003 | 8667 | 6479 | 15137 |
| Pakistan - SIUT [7][citation needed] | 1600 |
- Australian Aboriginal activist Charles Perkins, is the longest surviving Australian receiver of a kidney transplant, living twenty-eight years on his donor organ. [citation needed]
- Denice Lombard of Washington, D.C., received her father's kidney on August 30, 1967, aged 13 and is still alive and healthy forty years later.
- The Sindh Institute of Urology and Transplantation (SIUT) (siut.org SIUT) is the only hospital in the world giving free-of-cost transplantation treatment.[citation needed]
External links[edit | edit source]
- Template:MedlinePlusOverview
- med/3604 at eMedicine
- International Kidney Donors and Transplantations
- Living Donors Online
- Patient-oriented summary at emedicinehealth.com
- The Kidney Patient Guide
- 1/3/421
- Alliance for Paired Donation
References[edit | edit source]
- ↑ "Potential to replace race as a risk factor for kidney-transplant failure".
- ↑ "Medical Express".
- ↑ McDonald SP, Russ GR (2002). "Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991-2001". Nephrol. Dial. Transplant. 17 (12): 2212–9. PMID 12454235.
- ↑ "Facts and FAQs". Canada's National Organ and Tissue Information Site. Health Canada. 16 July 2002. Archived from the original on 2005-04-04. Retrieved 2007-01-06.
- ↑ 5.0 5.1 5.2 5.3 "European Activity Comparison 2003" (gif). UK Transplant. 2004. Retrieved 2007-01-06. Unknown parameter
|month=ignored (help) - ↑ "National Data Reports". The Organ Procurement and Transplant Network (OPTN). dynamic. Retrieved 2007-01-06. Check date values in:
|date=(help) (the link is to a query interface; Choose Category = Transplant, Organ = Kidney, and select the 'Transplant by donor type' report link) - ↑ Official Website of Sindh Instituite of Urology & Transplant
 KSF
KSF