Lipid
 From Wikidoc - Reading time: 13 min
From Wikidoc - Reading time: 13 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
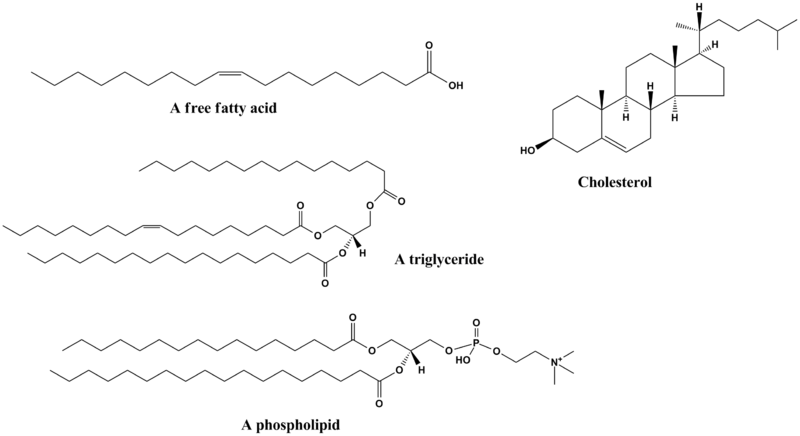
Lipids are broadly defined as any fat-soluble (lipophilic), naturally-occurring molecule, such as fats, oils, waxes, cholesterol, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The main biological functions of lipids include energy storage, acting as structural components of cell membranes, and participating as important signaling molecules.
Although the term lipid is sometimes used as a synonym for fats, fats are a subgroup of lipids called triglycerides and should not be confused with the term fatty acid. Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, and monoglycerides and phospholipids), as well as other sterol-containing metabolites such as cholesterol. [1]
Lipids are a diverse group of compounds that have many key biological functions, such as acting as structural components of cell membranes, serving as energy storage sources and participating in signaling pathways. Lipids may be broadly defined as hydrophobic or amphiphilic small molecules that originate entirely or in part from two distinct types of biochemical subunits or "building blocks": ketoacyl and isoprene groups.[2] Using this approach, lipids may be divided into eight categories : fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids and polyketides (derived from condensation of ketoacyl subunits); and sterol lipids and prenol lipids (derived from condensation of isoprene subunits).
Categories of Lipids[edit | edit source]

- Fatty acyls (including fatty acids) are a diverse group of molecules synthesized by chain-elongation of an acetyl-CoA primer with malonyl-CoA or methylmalonyl-CoA groups.[3][4] The fatty acyl structure represents the major lipid building block of complex lipids and therefore is one of the most fundamental categories of biological lipids. The carbon chain may be saturated or unsaturated, and may be attached to functional groups containing oxygen, halogens, nitrogen and sulfur. Examples of biologically interesting fatty acyls are the eicosanoids which are in turn derived from arachidonic acid which include prostaglandins, leukotrienes, and thromboxanes. Other major lipid classes in the fatty acyl category are the fatty esters and fatty amides. Fatty esters include important biochemical intermediates such as wax esters, fatty acyl thioester coenzyme A derivatives, fatty acyl thioester ACP derivatives and fatty acyl carnitines. The fatty amides include N-acyl ethanolamines such as anandamide.
- Glycerolipids are composed mainly of mono-, di- and tri-substituted glycerols,[5] the most well-known being the fatty acid esters of glycerol (triacylglycerols), also known as triglycerides. these comprise the bulk of storage fat in animal tissues. Additional subclasses are represented by glycosylglycerols, which are characterized by the presence of one or more sugar residues attached to glycerol via a glycosidic linkage. Examples of structures in this category are the digalactosyldiacylglycerols found in plant membranes and seminolipid from mammalian spermatazoa.
- Glycerophospholipids, also referred to as phospholipids, are ubiquitous in nature and are key components of the lipid bilayer of cells, as well as being involved in metabolism and signaling. Glycerophospholipids[6] may be subdivided into distinct classes, based on the nature of the polar headgroup at the sn-3 position of the glycerol backbone in eukaryotes and eubacteria or the sn-1 position in the case of archaebacteria. Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC or GPCho, and lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving as a primary component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of, or are themselves, membrane-derived second messengers. Typically one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in prokaryotes.
- Sphingolipids are a complex family of compounds[7] that share a common structural feature, a sphingoid base backbone that is synthesized de novo from serine and a long-chain fatty acyl CoA, then converted into ceramides, phosphosphingolipids, glycosphingolipids and other species. The major sphingoid base of mammals is commonly referred to as sphingosine. Ceramides (N-acyl-sphingoid bases) are a major subclass of sphingoid base derivatives with an amide-linked fatty acid. The fatty acids are typically saturated or mono-unsaturated with chain lengths from 14 to 26 carbon atoms. The major phosphosphingolipids of mammals are sphingomyelins (ceramide phosphocholines), whereas insects contain mainly ceramide phosphoethanolamines and fungi have phytoceramidephosphoinositols and mannose containing headgroups. The Glycosphingolipids are a diverse family of molecules composed of one or more sugar residues linked via a glycosidic bond to the sphingoid base. Examples of these are the simple and complex glycosphingolipids such as cerebrosides and gangliosides.
- Sterol lipids, such as cholesterol and its derivatives are an important component of membrane lipids,[8] along with the glycerophospholipids and sphingomyelins. The steroids, which also contain the same fused four-ring core structure, have different biological roles as hormones and signaling molecules. The C18 steroids include the estrogen family whereas the C19 steroids comprise the androgens such as testosterone and androsterone. The C21 subclass includes the progestogens as well as the glucocorticoids and mineralocorticoids. The secosteroids, comprising various forms of vitamin D, are characterized by cleavage of the B ring of the core structure. Other examples of sterols are the bile acids and their conjugates,[9] which in mammals are oxidized derivatives of cholesterol and are synthesized in the liver.

- Prenol lipids are synthesized from the 5-carbon precursors isopentenyl diphosphate and dimethylallyl diphosphate that are produced mainly via the mevalonic acid (MVA) pathway.[10] The simple isoprenoids (linear alcohols, diphosphates, etc.) are formed by the successive addition of C5 units, and are classified according to number of these terpene units. Structures containing greater than 40 carbons are known as polyterpenes. Carotenoids are important simple isoprenoids that function as anti-oxidants and as precursors of vitamin A. Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid core of non-isoprenoid origin. Vitamin E and vitamin K, as well as the ubiquinones, are examples of this class. Bacteria synthesize polyprenols (called bactoprenols) in which the terminal isoprenoid unit attached to oxygen remains unsaturated, whereas in animal polyprenols (dolichols) the terminal isoprenoid is reduced.
- Saccharolipids describe compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a sugar substitutes for the glycerol backbone that is present in glycerolipids and glycerophospholipids. The most familiar saccharolipids are the acylated glucosamine precursors of the Lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.[11]
- Polyketides are synthesized by polymerization of acetyl and propionyl subunits by classic enzymes as well as iterative and multimodular enzymes that share mechanistic features with the fatty acid synthases. They comprise a very large number of secondary metabolites and natural products from animal, plant, bacterial, fungal and marine sources, and have great structural diversity.[12] Many polyketides are cyclic molecules whose backbones are often further modified by glycosylation, methylation, hydroxylation, oxidation, and/or other processes. Many commonly used anti-microbial, anti-parasitic, and anti-cancer agents are polyketides or polyketide derivatives, such as erythromycins, tetracylines, avermectins, and antitumor epothilones.
Biological Functions[edit | edit source]
Membranes[edit | edit source]
The glycerophospholipids are the main structural component of biological membranes, such as the cellular plasma membrane and the intracellular membranes of organelles. In animal cells the plasma membrane physically separates the intracellular components from the extracellular environment. All eukaryotic cells are compartmentalized into membrane-bound organelles which carry out different functions. These glycerophospholipids are amphipathic molecules that contain a glycerol core linked to two fatty acid-derived "tails" by ester or, more rarely, ether linkages and to one "head" group by a phosphate ester linkage. While glycerophospholipids are the major component of biological membranes, other non-glyceride lipid components such as sphingomyelin and sterols (mainly cholesterol in animal cell membranes) are also found in biological membranes. In plants and algae, the galactosyldiacylglycerols,[13] and sulfoquinovosyldiacylglycerol,[14] which lack a phosphate group, are important components of membranes of chloroplasts and related organelles and are the most abundant lipids in photosynthetic tissues, including those of higher plants, algae and certain bacteria.

A biological membrane is a form of lipid bilayer, as is a liposome. The formation of lipid bilayers is an energetically-preferred process when the glycerophospholipids described above are in an aqueous environment. In an aqueous system, the polar heads of lipids orientate towards the polar, aqueous environment, while the hydrophobic tails minimise their contact with water. The lipophilic tails of lipids (U) tend to cluster together, forming a lipid bilayer (1) or a micelle (2). Other aggregations are also observed and form part of the polymorphism of amphiphile (lipid) behaviour. The polar heads (P) face the aqueous environment, curving away from the water. Phase behaviour is a complicated area within biophysics and is the subject of current academic research. Micelles and bilayers form in the polar medium by a process known as the hydrophobic effect.[15] When dissolving a lipophilic or amphiphilic substance in a polar environment, the polar molecules (i.e. water in an aqueous solution) become more ordered around the dissolved lipophilic substance, since the polar molecules cannot form hydrogen bonds to the lipophilic areas of the amphiphile. So in an aqueous environment the water molecules form an ordered "clathrate" cage around the dissolved lipophilic molecule.[16]
Energy storage and metabolism[edit | edit source]
Triacylglycerols, stored in adipose tissue, are a major form of energy storage in animals. Animals use triglycerides for energy storage because of its high caloric content (9 KCal/g), whereas plants, which do not require energy for movement, can afford to store food for energy in a less compact but more easily accessible form, such as starch (carbohydrate). Triglycerides and phospholipids are broken down into free fatty acids by the action of lipases. Beta oxidation is the process by which fatty acids, in the form of acyl-CoA molecules, are broken down in the mitochondria and/or in peroxisomes to generate acetyl-CoA. The acetyl CoA is then ultimately converted into ATP, CO2, and H2O using the citric acid cycle and the electron transport chain. Conversely, fatty acid biosynthesis (Lipogenesis) takes place in the cytoplasm, using acetyl-CoA (derived from carbohydrates, amino acids or fatty acids) as the precursor[17]. The fatty acids may be subsequently converted to triacylglycerols that are packaged in lipoproteins (VLDL's) and secreted from the liver.
Signaling[edit | edit source]
In recent years, evidence has emerged showing that lipid signaling is a vital part of the cell signaling.[18] Lipid signaling may occur via activation of GPCR's or nuclear receptors, and members of several different lipid categories have been identified as signaling molecules and cellular messengers.[19] These include sphingosine-1-phosphate, a sphingolipid derived from ceramide that is a potent messenger molecule involved in regulating calcium mobilization, cell growth, apoptosis; diacylglycerol(DAG) and the phosphatidylinositol phosphates (PIPs), involved in calcium-mediated activation of protein kinase C; the prostaglandins, arachidonic acid -derived fatty acids involved in inflammation and immunity; the steroid hormones such as estrogen, testosterone and cortisol, which modulate a host of functions such as reproduction, metabolism and blood pressure; and the oxysterols such as 25-hydroxy-cholesterol that are Liver X receptor (LXR) agonists.
Other functions[edit | edit source]
The "fat-soluble" vitamins (A, D, E and K) which are isoprene-based lipids are essential nutrients stored in the liver and fatty tissues. These have a diverse range of functions discussed elsewhere. Acyl-carnitines are involved in the transport and metabolism of fatty acids in and out of mitochondria, where they undergo beta oxidation. Polyprenols and their phosphorylated derivatives also play important transport roles, in this case the transport of oligosaccharides across membranes. Polyprenol phosphate sugars and polyprenol diphosphate sugars function in extra-cytoplasmic glycosylation reactions, in extra-cellular polysaccharide biosynthesis (for instance peptidoglycan polymerization in bacteria), and in eukaryotic protein N-glycosylation.[20] Cardiolipins are a subclass of glycerophospholipids containing four acyl chains and three glycerol groups that are particularly abundant in the inner mitochondrial membrane. They are believed to activate enzymes involved with oxidative phosphorylation.[21]
Nutrition and health[edit | edit source]
Lipids play diverse and important roles in nutrition and health.[22] Many lipids are absolutely essential for life. However, there is also considerable awareness that abnormal levels of certain lipids, particularly cholesterol (in hypercholesterolemia) and trans fatty acids, are risk factors for heart disease amongst others.
Humans have a requirement for certain essential fatty acids, such as linoleic acid (an omega-6 fatty acid) and alpha-linolenic acid (an omega-3 fatty acid) in the diet because they cannot be synthesized from simple precursors in the diet. Both of these fatty acids are 18-carbon polyunsaturated fatty acids differing in the number and position of the double bonds. Most vegetable oils are rich in linoleic acid (safflower, sunflower, and corn oils). Alpha-linolenic acid is found in the green leaves of plants, and in selected seeds, nuts and legumes (flax, canola, walnuts and soy). Fish oils are particularly rich in the longer-chain omega-6 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Most of the lipid found in food is in the form of triacylglycerols, cholesterol and phospholipids.
Most of the saturated fatty acids (as triacylglycerols) in the diet are incorporated into adipose tissue stores, because the absence of double bonds allows a higher energy yield per carbon than is obtained from oxidation of unsaturated fatty acids. The longer chain fatty acids are incorporated into cell membranes as phospholipids regardless of degree of saturation. Since dietary fatty acids are exchanged with membrane fatty acids, dietary fat composition is reflected in membrane lipid composition. Thus dietary fatty acids can influence cell function through effects on membrane properties. Dietary fat provides an average energy intake which is approximately twice that of carbohydrate or protein. A minimum amount of dietary fat is necessary to facilitate absorption of fat-soluble vitamins (A, D, E and K) and carotenoids. A minimal amount of body fat is also necessary to provide insulation that prevents heat loss and protects vital organs from shock due to ordinary activities.
High fat intake contributes to increased risk of obesity, diabetes and atherosclerosis. Atherosclerosis is the primary cause of coronary and cardiovascular diseases and is primary due to the buildup of plaque on the inside walls of arteries. Plaque is made up of cholesterol-rich low density lipoproteins (LDL), macrophages, smooth muscle cells, platelets, and other substances. In North America and most other western countries, atherosclerosis is the leading cause of illness and death, almost doubling the number of deaths from cancers. Despite significant medical advances, coronary artery disease and atherosclerotic stroke are responsible for more deaths than all other causes combined. A substantial amount of scientific evidence supports the impact of dietary fatty acids on cardiovascular health. Saturated fats have a profound hypercholesterolemic (increase blood cholesterol levels) effect and tend to increase plasma LDL. They are found predominantly in animal products (butter, cheese and meat) but coconut oil and palm oil are common vegetable sources. Intake of monounsaturated fats in oils such as olive oil is thought to be preferable to consumption of polyunsaturated fats in oils such as corn oil because the monounsaturated fats apparently do not lower high-density-lipoprotein (HDL) cholesterol levels.[23] Keeping cholesterol in the normal range not only helps prevent heart attacks and strokes but may also prevent the progression of atherosclerosis. "Statins" are a class of drugs that lowers the level of cholesterol in the blood by inhibiting the enzyme HMG-CoA reductase. This is a key enzyme involved in the biosynthesis of cholesterol in the liver.
References[edit | edit source]
- ↑ Maton, Anthea (1993). Human Biology and Health. Englewood Cliffs, New Jersey, USA: Prentice Hall. ISBN 0-13-981176-1. Unknown parameter
|coauthors=ignored (help) - ↑ Fahy E, Subramaniam S, Brown HA; et al. (2005). "A comprehensive classification system for lipids". J. Lipid Res. 46 (5): 839–61. PMID 15722563.
- ↑ Vance, Jean E.; Vance, Dennis E. (2002). Biochemistry of lipids, lipoproteins and membranes. Amsterdam: Elsevier. ISBN 0444511393.
- ↑ Lipodomics and Bioactive Lipids: Mass Spectrometry Based Lipid Analysis, Volume 432 (Methods in Enzymology). Boston: Academic Press. ISBN 0123738954.
- ↑ Coleman, R.A. and Lee, D.P. (2004). "Enzymes of triacylglycerol synthesis and their regulation". Prog. Lipid Res. 43: 134–176.
- ↑ Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA (2007). "Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry". Meth. Enzymol. 432: 21–57. doi:10.1016/S0076-6879(07)32002-8. PMID 17954212.
- ↑ Merrill, A.H., Jr. and Sandhoff, K.(2002) "Sphingolipids: metabolism and cell signaling",in New Comprehensive Biochemistry: Biochemistry of Lipids, Lipoproteins,and Membranes, Vance, D.E. and Vance, J.E., eds. Elsevier Science, NY. Ch. 14.
- ↑ Bach, D., and Wachtel, E. (2003). "Phospholipid/cholesterol model membranes: formation of cholesterol crystallites". Biochim Biophys Acta. 1610: 187–197.
- ↑ Russell, D.W. (2003). "The enzymes, regulation, and genetics of bile acid synthesis". Annu.Rev Biochem. 72: 137–174.
- ↑ Kuzuyama, T. and Seto, H. (2003). "Diversity of the biosynthesis of the isoprene units". Nat.Prod Rep. 20: 171–183.
- ↑ Raetz, C. R., Garrett, T. A., Reynolds, C. M.; et al. (2006). "Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4". J. Lipid Res. 47: 1097–1111. PMID 16479018.
- ↑ Walsh, C.T. (2004). "Polyketide and nonribosomal peptide antibiotics: modularity and versatility". Science. 303: 1805–1810.
- ↑ Heinz, E.(1996) Plant glycolipids: structure, isolation and analysis. in Advances in Lipid Methodology - 3, pp. 211-332 (ed. W.W. Christie, Oily Press, Dundee)
- ↑ Hölzl, G. and Dörmann, P. (2007). "Structure and function of glycoglycerolipids in plants and bacteria". Prog. Lipid Res. 46: 225–243.
- ↑ Wiggins PM (1990). "Role of water in some biological processes". Microbiol. Rev. 54 (4): 432–49. PMID 2087221.
- ↑ Raschke TM, Levitt M (2005). "Nonpolar solutes enhance water structure within hydration shells while reducing interactions between them". Proc. Natl. Acad. Sci. U.S.A. 102 (19): 6777–82. PMID 15867152.
- ↑ Berg, J.M., Tymoczko, J.L., Stryer, L.(2006) Biochemistry, Freeman, New York.5th ed. ISBN 0716787245
- ↑ Wang X (2004). "Lipid signaling". Curr. Opin. Plant Biol. 7 (3): 329–36. PMID 15134755.
- ↑ Eyster,K.M. (2007). "The membrane and lipids as integral participants in signal transduction". Adv. Physiol. Edu. 31: 5–16.
- ↑ Helenius, A., Aebi, M. (2001). "Intracellular functions of N-linked glycans". Science. 291: 2364–2369. PMID 11269317.
- ↑ Hoch, FL (1992). "Cardiolipins and biomembrane function". Biochim. Biophys. Acta. 1113 (1): 71–133. PMID 10206472.
- ↑ Spiller, G.A. ed., (2006), Handbook of Lipids in Human Nutrition,Boca Raton: CRC Press.
- ↑ Dreon, D.M., Vranizan, K.M., Krauss, R.M., Austin, M.A., Wood, P.D. (1990). "The effects of polyunsaturated fat vs monounsaturated fat on plasma lipoproteins". JAMA. 263 (18): 2462–6. PMID 2329634.
See also[edit | edit source]
| Wikimedia Commons has media related to Lipids. |
External links[edit | edit source]
Introductory
- Lipid Library - General reference on lipid chemistry and biochemistry
- European Lipidomics Initiative - European research resource for lipid biochemistry.
- Cyberlipid.org - Resources and history for lipids.
- Science aid: Lipids - Resource for students
- Molecular Computer Simulations - Modeling of Lipid Membranes
- Lipids, Membranes and Vesicle Trafficking - The Virtual Library of Biochemistry and Cell Biology
Classification
- LIPID MAPS - LIPID Metabolites And Pathways Strategy
Nomenclature
Databases
- LIPID MAPS - Comprehensive lipid and lipid-associated gene/protein databases.
- LipidBank - Japanese database of lipids and related properties, spectral data and references.
- LIPIDAT - Database composed mainly of phospholipids and associated thermodynamic data.
General
- ApolloLipids - Provides dyslipidemia and cardiovascular disease prevention and treatment information as well as continuing medical education programs
Template:Food chemistry Template:Lipids
Template:Phospholipids Template:Sphingolipids
Template:Link FA ar:ليبيدات bg:Липид ca:Lípid cs:Lipidy da:Lipid de:Lipide eo:Lipido eu:Lipido fa:لیپید fi:Lipidi he:ליפיד id:Lipid is:Fita it:Lipidi ko:지질 hr:Lipidi lb:Lipid lt:Lipidai lv:Lipīdi mk:Липид nl:Lipide no:Lipid pam:Lipid sh:Lipidi simple:Lipid sk:Lipid sl:Lipid sr:Липиди su:Lipid sv:Lipid th:ไลปิด uk:Ліпіди
 KSF
KSF