Lysergic acid diethylamide detailed information
 From Wikidoc - Reading time: 28 min
From Wikidoc - Reading time: 28 min
 | |
| Clinical data | |
|---|---|
| Synonyms | LSD, LSD-25, lysergide, D-lysergic acid diethylamide, N,N-diethyl-D-lysergamide |
| Pregnancy category | |
| Routes of administration | Oral, Intravenous, Transdermal |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | hepatic |
| Elimination half-life | 3 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C20H25N3O |
| Molar mass | 323.431 g/mol |
| 3D model (JSmol) | |
| Melting point | 80 °C (176 °F) |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]
Overview[edit | edit source]
Lysergic acid diethylamide, LSD, LSD-25, or acid, is a semisynthetic psychedelic drug of the ergoline family. Probably the most widely known psychedelic, it has been used mainly as a recreational drug, an entheogen, and a tool to supplement various practices for transcendence, including in meditation, psychonautics, art projects, and illicit (though at one time legal) psychedelic therapy, whether self-administered or not. It is synthesized from lysergic acid derived from ergot, a grain fungus that typically grows on rye and was first synthesized by Swiss chemist Albert Hofmann. The short form LSD comes from its early codename LSD-25, which is an abbreviation for the German "Lysergsäure-diethylamid" followed by a sequential number.[1][2]
LSD is sensitive to oxygen, ultraviolet light, and chlorine, especially in solution, though its potency may last for years if it is stored away from light and moisture at low temperature. In pure form it is colorless, odorless, and mildly bitter.[2] LSD is typically delivered orally, usually on a substrate such as absorbent blotter paper, a sugar cube, or gelatin. In its liquid form, it can be administered by intramuscular or intravenous injection. The threshold dosage level needed to cause a psychoactive effect on humans is of the order of 20 to 30 µg (micrograms).
Introduced by Sandoz Laboratories as a drug with various psychiatric uses, LSD quickly became a therapeutic agent that appeared to show great promise. However, the extra-medicinal use of the drug in Western society during the mid-twentieth century led to a political firestorm that resulted in the banning of the substance.[3] A number of organizations—including the Beckley Foundation, MAPS, Heffter Research Institute and the Albert Hofmann Foundation—exist to fund, encourage and coordinate research into its medicinal uses.[4]
History[edit | edit source]
LSD was first synthesized on November 16, 1938 by a Swiss chemist named Dr. Albert Hofmann at the Sandoz Laboratories in Basel, Switzerland, as part of a large research program searching for medically useful ergot alkaloid derivatives.[5] Ergot is a fungus that, by infecting cereal grains used for making rye breads, causes ergotism. After Dr. Hofmann succeeded in synthesizing ergobasine (which became the preeminent uterotonic), he began working on other amide derivatives of lysergic acid. LSD (lysergic acid diethylamide) is one of the major drugs making up the hallucinogen class of drugs.[6] Lysergic acid diethylamide, the 25th lysergic acid derivative Hofmann synthesised (hence the name LSD-25) was developed initially as a probable analeptic, a circulatory and respiratory stimulant, based on its structural similarity to another known analeptic, nikethamide (nicotinic acid diethylamide). However, no extraordinary benefits of the compound were identified during animal tests (though laboratory notes briefly mention that the animals became "restless" under its effects), and its study was discontinued.[7] Its psychedelic properties were unknown until 5 years later, when Hofmann, acting on what he has called a "peculiar presentiment," returned to work on the chemical.[7] While re-synthesizing LSD-25 for further study on April 16 1943, Hofmann became dizzy and was forced to stop work. In his journal, Hofmann wrote that after becoming dizzy he proceeded home and was affected by a "remarkable restlessness, combined with a slight dizziness". Hofmann stated that as he lay in his bed he sank into a not unpleasant "intoxicated like condition" which was characterized by an extremely stimulated imagination. He stated that he was in a dreamlike state, and with his eyes closed he could see uninterrupted streams of "fantastic pictures, extraordinary shapes with intense, kaleidoscopic play of colors." The condition lasted about two hours after which it faded away.[8] Hofmann had attributed the psychoactive effects he experienced to accidentally absorbing a tiny amount of LSD-25 into his skin. Three days later he would take a much larger dose in order to test its effects further; this day would later be referred to as the "Bicycle Day".[1]
Bicycle day[edit | edit source]
On April 19, 1943 Dr. Albert Hofmann intentionally ingested 250 µg of LSD, which he hypothesized would be at most a threshold level dose, based on his research on other ergot alkaloids. Surprisingly, the substance showed a potency orders of magnitude above almost any other substance known at the time, amounting to a much heavier dose than typically given in modern therapeutic use. After ingesting the substance Hofmann found himself struggling to speak intelligibly and asked his laboratory assistant, who knew of the self-experiment, to escort him home on his bicycle, since wartime restrictions made automobiles unavailable. On the bicycle ride home, Hofmann's condition became more severe and in his journal he stated that everything in his field of vision wavered and was distorted, as if seen in a curved mirror. Hofmann also stated that while riding on the bicycle, he had the sensation of being stationary, unable to move from where he was, despite the fact that he was moving very rapidly. Once Hofmann arrived home, he summoned a doctor and asked his neighbor for milk, believing it might help relieve the symptoms. Hofmann wrote that despite his delirious and bewildered condition, he was able to choose milk as a nonspecific antidote for poisoning.[9] Upon arriving the attending doctor could find no abnormal physical symptoms other than extremely dilated pupils. After spending several hours terrified that his body had been possessed by a demon, that his next door neighbor was a witch, and that his furniture was threatening him, Dr. Hofmann feared he had become completely insane. In his journal Hofmann said that the doctor saw no reason to prescribe medication and instead sent him to his bed. At this time Hofmann said that the feelings of fear had started to give way to feelings of good fortune and gratitude, and that he was now enjoying the colors and plays of shapes that persisted behind his closed eyes. Hofmann mentions seeing "fantastic images" surging past him, alternating and opening and closing themselves into circles and spirals and finally exploding into colored fountains and then rearranging themselves in a constant flux. Hofmann mentions that during the condition every acoustic perception, such as the sound of a passing automobile, was transformed into optical perceptions. Eventually Hofmann slept and upon awakening the next morning felt refreshed and clearheaded, though somewhat physically tired. He also stated that he had a sensation of well being and renewed life and that his breakfast tasted unusually delicious. Upon walking in his garden he remarked that all of his senses were "vibrating in a condition of highest sensitivity, which then persisted for the entire day".[9]
Early research[edit | edit source]
Early researchers on LSD saw its potency and noticed that even in extremely small quantities it could significantly alter the mental functioning of healthy volunteers. Due to the fact that LSD could produce changes in perceptions and emotions, early researchers hypothesized that the cause of some mental illnesses, particularly schizophrenia, were due to the human body releasing small quantities of substances identical to LSD.[10] Much of the research during the late 1940s dealt with this hypothesis and many LSD sessions conducted for scientific study were often termed "experimental psychoses", and this is where the terms "psychoactive" , "psychotomimetic" and "hallucinogenic" were coined to refer to such drugs. Generally these studies revolved around the attempt to block the effects of LSD with premedication, which was thought to be able to lead to medical treatments for schizophrenia. The studies showed that there was no such connection (the effects of LSD and those of schizophrenia are drastically different and have different causes and functions). Some early researchers also started to suggest that LSD could have positive effects and could be used as a treatment for patients with psychiatric illnesses. Some reports suggested that even small doses of LSD could have dramatic effects on the personalities and attitudes and even lifestyles of test subjects. Early LSD research also found evidence of the drug's ability to facilitate relief of various emotional episodes related to traumatic memories from childhood of patients.[10]
Government research[edit | edit source]

During the Cold War, intelligence agencies were keenly interested in the possibilities of using LSD for interrogation and mind control, as well as for large-scale social engineering. The CIA research on LSD, most of which was done under Project MKULTRA, the code name for a CIA mind-control research program, began in the 1950s and continued until the late 1960s.[11]
Tests were also conducted by the U.S. Army Biomedical Laboratory (now known as the U.S. Army Medical Research Institute of Chemical Defense) located in the Edgewood Arsenal at Aberdeen Proving Grounds. The government would administer LSD to subjects (without consent) and then perform a battery of tests to investigate the effects of the drug on soldiers. Based on remaining publicly available records, the projects seem to have concluded that LSD was of little practical use as a mind control drug and moved on to other drugs.[12]
Both the CIA and the Army experiments became highly controversial when they became public knowledge in the 1970s, as the test subjects were not normally informed of the nature of the experiments, or even that they were subjects in experiments at all.[11] In 1961, Paul Robeson attempted suicide in a Moscow hotel room. His son claimed this was precipitated by a CIA agent who placed some synthetic hallucinogen in his drink.[5] At least one person, an Army scientist named Frank Olson is thought by some to have committed suicide by leaping from a tall building as a result of his being unknowingly given LSD.[11] Frank Olson's son, Eric Olson, believes that his father was murdered by government officials and a 1994 exhumation and examination by forensic pathologists at George Washington University of the body suggested that Olson had suffered blunt trauma to the back of his head prior to falling from the building.[13] Most of the MKULTRA records were deliberately destroyed in 1973. The controversy contributed to President Ford's creation of the Rockefeller Commission and new regulations on informed consent.[11]The British government also engaged in LSD testing; in 1953 and 1954, scientists working for MI6 dosed servicemen in an effort to find a "truth drug". The test subjects were not informed that they were being given LSD, and had in fact been told that they were participating in a medical project to find a cure for the common cold. One subject, aged 19 at the time, reported seeing "walls melting, cracks appearing in people's faces … eyes would run down cheeks, Salvador Dalí-type faces … a flower would turn into a slug". After keeping the trials secret for many years, MI6 agreed in 2006 to pay the former test subjects financial compensation. Like the CIA, MI6 decided that LSD was not a practical drug for mind control purposes.[14]
Current research[edit | edit source]
Today, most research with LSD involves animals or cells. However, a few groups are exploring LSD effects in humans. The Multidisciplinary Association for Psychedelic Studies has an eight-person study in Switzerland to see if a large dose of LSD (200 ug) is more helpful as part of psychotherapy for cancer patients than a lower dose (20 ug). The Beckley Foundation is studying the effects of LSD on mental activity and consciousness in LSD-experienced volunteers, in order to gain insight into its reported effects on creativity and insight.[15] There has been additional interest in studying the effects of LSD on cluster headaches, although the current status of this research is uncertain. While these studies could be criticized for being too small to lead to strong conclusions, they may represent the beginnings of renewed scientific interest into LSD.
Dosage[edit | edit source]
Dosages of LSD are measured in micrograms (µg), or millionths of a gram. By comparison, dosages of almost all other drugs, both recreational and medicinal, are measured in milligrams (mg), or thousandths of a gram. Hofmann determined that an active dose of mescaline, roughly 0.2 to 0.5g, has effects comparable to 100µg or less of LSD; put another way, LSD is between five to ten thousand times more active than mescaline.[1]
While a single dose of LSD may be between 100 and 500 micrograms — an amount roughly equal to one-tenth the mass of a grain of sand — threshold effects can be felt with as little as 25 micrograms.[16]
Generally, the dosage that will produce a threshold psychotropic effect in humans is considered to be 20 to 30µg.[17][16] According to Glass and Henderson's review, black-market LSD is largely iterated though sometimes contaminated by manufacturing by-products. Typical doses in the 1960s ranged from 200 to 1000µg while street samples of the 1970s contained 30 to 300µg. By the 1980s, the amount had reduced to between 100 to 125 µg, lowering more in the 1990s to the 20–80 µg range. (Lower doses, Glass and Henderson found, generally produce fewer bad trips.)[18]
Estimates for the lethal dosage (LD50) of LSD range from between 200 µg/kg to more than 1 mg/kg of human body mass, though most sources report that there are no known human cases of such an overdose. Other sources note one report of a suspected fatal overdose of LSD occurring in November 1975 in Kentucky in which there were indications that ~1/3 of a gram (320 mg or 320,000 µg) had been injected intravenously, i.e., over 3,000 more typical oral doses of ~100 µg had been injected.[19][20]
Tusko the elephant died shortly after being injected with 297 mg in 1962, but whether the LSD was the cause of his death is controversial.
LSD is not considered addictive, in that its users do not exhibit the medical community's commonly accepted definitions of addiction and physical dependence. Rapid tolerance build-up prevents regular use, and there is cross-tolerance shown between LSD, mescaline[21] and psilocybin.[22] This tolerance diminishes after a few days without use and is probably caused by downregulation of 5-HT2A receptors in the brain.
Adverse effects of psychotropics are often treated with fast acting benzodiazepines like diazepam or triazolam that have calming and antianxiety effects but do not directly affect the specific actions of psychotropics. Many rumors about home remedies to counteract psychedelic effects are circulated, including sugar, calcium, orange juice, milk, or niacin, but none of them have been shown to be effective and they make no sense from a pharmacological standpoint. Theoretically, specific 5-HT2A receptor antagonists, such as Seroquel, would be direct antidotes, although some anecdotal reports claim otherwise.[23] Also, some people have reported that taking a SSRI such as Prozac or drugs that are 5-HT2 receptor antagonists such as Trazodone will counteract the effects of LSD.
Effects[edit | edit source]
Pharmacokinetics[edit | edit source]

LSD's effects normally last from 6-12 hours depending on dosage, tolerance, body weight and age[2] - Sandoz's prospectus for "Delysid" warned: "intermittent disturbances of effect may occasionally persist for several days."[1] Contrary to early reports and common belief, LSD effects do not last longer than significant levels of the drug in the blood. Aghajanian and Bing found LSD had an elimination half-life of 175 minutes,[24] while, more recently, Papac and Foltz reported that 1 µg/kg oral LSD given to a single male volunteer had an apparent plasma half-life of 5.1 hours, with a peak plasma concentration of 5 ng/mL at 3 hours post-dose.[25] Notably, Aghajanian and Bing found that blood concentrations of LSD matched the time course of volunteers' difficulties with simple arithmetic problems.
Pharmacodynamics[edit | edit source]
LSD affects a large number of the G protein coupled receptors, including all dopamine receptor subtypes, all adrenoreceptor subtypes as well as many others. LSD binds to most serotonin receptor subtypes except for 5-HT3 and 5-HT4. However, most of these receptors are affected at too low affinity to be activated by the brain concentration of approximate 10–20 nM.[26] Recreational doses of LSD can affect 5-HT1A, 5-HT2A, 5-HT2C, 5-HT5A, 5-HT5B, and 5-HT6 receptors. The psychotropic effects of LSD are attributed to its strong partial agonist effects at 5-HT2A receptors as specific 5-HT2A agonist drugs are psychotropics and largely 5-HT2A specific antagonists block the psychotropic activity of LSD.[26] Exactly how this produces the drug's effects is unknown, but it is thought that it works by increasing glutamate release and hence excitation in the cortex, specifically in layers IV and V.[27] In the later stages, LSD might act through DARPP-32 - related pathways that are likely the same for multiple drugs including cocaine, methamphetamine, nicotine, caffeine, PCP, ethanol and morphine.[28]
One experiment studying the actions of LSD was performed by Barry Jacobs recording from electrodes implanted into cat Raphe nuclei.[29] Behaviorally relevant doses of LSD result in a complete blockade of action potential activity in the dorsal raphe, effectively shutting off the principal endogenous source of serotonin to the telencephalon.

Some reports indicate that although administration of chlorpromazine (Thorazine) or similar typical antipsychotic tranquilizers will not end an LSD trip, it will either lessen the intensity or immobilize and numb the patient, a side effect of the medication.[30] While it also may not end an LSD trip, the best chemical treatment for a "bad trip" is an anxiolytic agent such as diazepam (Valium) or another benzodiazepine. Some have suggested that administration of niacin (nicotinic acid, vitamin B3) could be useful to end the LSD user's experience of a "bad trip".[31] The nicotinic acid in niacin as opposed to nicotinamide, will produce a full body heat rash, due to widening of peripheral blood vessels. The effect is somewhat akin to a poison ivy rash. Although it is not clear to what extent the effects of LSD are reduced by this intervention, the physical effect of an itchy skin rash may itself tend to distract the user from feelings of anxiety. Indeed, nicotinic acid was experienced as a stressor by all tested persons. The rash itself is temporary and disappears within a few hours. It is questionable if this method could be effective for people having serious adverse psychological reactions.
Physical[edit | edit source]
Physical reactions to LSD are highly variable and may include the following: uterine contractions, hypothermia, fever, elevated levels of blood sugar, goose bumps, increase of heart rate, jaw clenching, perspiration, pupil-dilation, saliva production, mucus production, sleeplessness, paresthesia, euphoria, hyperreflexia, tremors and synesthesia. LSD users report numbness, weakness, trembling, and nausea.[32] LSD was studied in the 1960s by Eric Kast as an analgesic for serious and chronic pain caused by cancer or other major trauma.[33] Even at low (sub-psychedelic) dosages, it was found to be at least as effective as traditional opiates while being much longer lasting (pain reduction lasting as long as a week after peak effects had subsided). Kast attributed this effect to a decrease in anxiety. This reported effect is being tested (though not using LSD) in an ongoing (as of 2006) study of the effects of the psychedelic tryptamine psilocybin on anxiety in terminal cancer patients.
Furthermore, LSD has been used as a treatment for cluster headaches, an uncommon but extremely painful disorder. Researcher Peter Goadsby describes the headaches as "worse than natural childbirth or even amputation without anesthetic."[34] Although the phenomenon has not been formally investigated, case reports indicate that LSD and psilocybin can reduce cluster pain and also interrupt the cluster-headache cycle, preventing future headaches from occurring. Currently existing treatments include various ergolines, among other chemicals, so LSD's efficacy may not be surprising. A dose-response study, testing the effectiveness of both LSD and psilocybin was planned at McLean Hospital, although the current status of this project is unclear. A 2006 study by McLean researchers interviewed 53 cluster-headache sufferers who treated themselves with either LSD or psilocybin, finding that a majority of the users of either drug reported beneficial effects.[35] Unlike attempts to use LSD or MDMA in psychotherapy, this research involves non-psychological effects and often sub-psychedelic dosages; therefore, it is plausible that a respected medical use of LSD will arise.[36]
Psychological[edit | edit source]
LSD's psychological effects (colloquially called a "trip") vary greatly from person to person, depending on factors such as previous experiences, state of mind and environment, as well as dose strength. They also vary from one trip to another, and even as time passes during a single trip. An LSD trip can have long term psychoemotional effects; some users cite the LSD experience as causing significant changes in their personality and life perspective. Widely different effects emerge based on what has been called set and setting; the "set" being the general mindset of the user, and the "setting" being the physical and social environment in which the drug's effects are experienced.
Timothy Leary and Richard Alpert considered the chemical to be of potentially beneficial application in psychotherapy. If the user is in a hostile or otherwise unsettling environment, or is not mentally prepared for the powerful distortions in perception and thought that the drug causes, effects are more likely to be unpleasant than if he or she is in a comfortable environment and has a relaxed, balanced and open mindset.
Some psychological effects may include an experience of radiant colors, objects and surfaces appearing to ripple or "breathe," colored patterns behind the eyes, a sense of time distorting (time seems to be stretching, repeating itself, changing speed or stopping), crawling geometric patterns overlaying walls and other objects, morphing objects, a sense that one's thoughts are spiraling into themselves, loss of a sense of identity or the ego (known as "ego death"), and powerful, and sometimes brutal, psycho-physical reactions interpreted by some users as reliving their own birth.[10][37]
Many users experience a dissolution between themselves and the "outside world".[38] This unitive quality may play a role in the spiritual and religious aspects of LSD. The drug sometimes leads to disintegration or restructuring of the user's historical personality and creates a mental state that some users report allows them to have more choice regarding the nature of their own personality.
Some experts hypothesize that drugs such as LSD may be useful in psychotherapy, especially when the patient is unable to "unblock" repressed subconscious material through other psychotherapeutic methods,[39] and also for treating alcoholism. One study concluded, "The root of the therapeutic value of the LSD experience is its potential for producing self-acceptance and self-surrender,"[40] presumably by forcing the user to face issues and problems in that individual's psyche. Many believe that, in contrast, other drugs (such as alcohol, heroin, and cocaine) which are used to escape from reality, LSD is seen as more of an introspective experience. Studies in the 1950s that used LSD to treat alcoholism professed a 50% success rate,[41] five times higher than estimates near 10% for Alcoholics Anonymous.[42]
Some LSD studies were criticized for methodological flaws, and different groups had inconsistent results. Mangini's 1998 paper reviewed this history. He concluded that the efficacy of LSD in treating alcoholism remains an open question.[43] Dr Abram Hoffer referred to Mangini's paper as "a good review of the literature" but said that, in common with many other scientists, the author has failed to grasp the important point that psychedelic therapy is a therapeutic experience.
The critics of psychedelic therapy have not taken this into account. Thus the Toronto studies studied the drug. They made no attempt whatever to induce a psychedelic experience. I saw at least two of the patients many years after they had been treated in Toronto and they told me that it was the most horrible experience they had ever had. It was in fact a true psychotomimetic experience and probably reproduced delirium tremens more than anything else. Not surprisingly their patients did not do well. They gave them 800 micrograms which is too heavy, gave them a barbiturate in advance to prevent convulsions, tied them to the bed so that they could not run away, and had sitting with them a psychologist who wrote notes all the time and did not interact with the patients.
— Abram Hoffer M.D, Ph.D, FRCP, Comments on the article Treatment of Alcoholism Using Psychedelic Drugs
Many notable individuals have commented publicly on their experiences with LSD. Some of these comments date from the era when it was legally available in the US and Europe for non-medical uses, and others pertain to psychiatric treatment in the 1950s and 60s. Still others describe experiences with illegal LSD, obtained for philosophic, artistic, therapeutic, spiritual, or recreational purposes.
Sensory / perception[edit | edit source]
LSD causes expansion and altered experience of senses, emotions, memories, time, and awareness for 6 to 14 hours, depending on dosage and tolerance. LSD does typically not produce real hallucinations as the deliriants do. Generally beginning within thirty to ninety minutes after ingestion, the user may experience anything from subtle changes in perception to overwhelming cognitive shifts. Changes in auditory and visual perception are typical.[38][44] Visual effects include the illusion of movement of static surfaces ("walls breathing"), after image-like trails of moving objects ("tracers"), the appearance of moving colored geometric patterns (especially with closed eyes), an intensification of colors and brightness ("sparkling"), new textures on objects, blurred vision, and shape suggestibility. Users commonly report that the inanimate world appears to animate in an unexplained way; for instance, objects that are static in three dimensions can seem to be moving relative to one or more additional spatial dimensions.[45] Many of the basic visual effects resemble the phosphenes seen after applying pressure to the eye and have also been studied under the name "form constants". The auditory effects of LSD include echo-like distortions of sounds, a mixing of all sounds which makes it harder to discern distinct sounds, the feeling that what you're hearing is your thought, a general intensification of the experience of music, and an increased discrimination of instruments and sounds. Higher doses often cause intense and fundamental distortions of sensory perception such as synaesthesia, the experience of additional spatial or temporal dimensions, and temporary dissociation.
Spiritual[edit | edit source]
LSD is considered an entheogen because it can catalyze intense spiritual experiences where users feel they have come into contact with a greater spiritual or cosmic order. Some users report insights into the way the mind works, and some experience long-lasting changes in their life perspective. Some users consider LSD a religious sacrament, or a powerful tool for access to the divine. Dr. Stanislav Grof has written that religious and mystical experiences observed during LSD sessions appear to be phenomenologically indistinguishable from similar descriptions in the sacred scriptures of the great religions of the world and the secret mystical texts of ancient civilizations.[46]
Such experiences under the influence of LSD have been observed and documented by researchers such as Alan Watts, Timothy Leary and Stanislav Grof. For example, Walter Pahnke conducted the Good Friday Marsh Chapel Experiment in 1962 under Leary's supervision, performing a double blind experiment on the administration of psilocybin to volunteers who were students in religious graduate programs, e.g., divinity or theology.[47] That study provided evidence that psychotropics may induce mystical religious states.[48]
Potential risks of LSD use[edit | edit source]
LSD is generally considered nontoxic; it may temporarily impair the ability to make sensible judgments and understand common dangers, thus making the user more susceptible to accidents and personal injury.
There is also some indication that LSD may trigger a dissociative fugue state in individuals who are taking certain classes of antidepressants such as lithium salts and tricyclics. In such a state, the user has an impulse to wander, and may not be aware of his or her actions, which can lead to physical injury.[49] SSRIs are believed to interact more benignly, with a tendency to noticeably reduce LSD's subjective effects.[50] Similar and perhaps greater reductions have also been reported with MAOIs.[49]
As Albert Hofmann reports in LSD – My Problem Child, the early pharmacological testing Sandoz performed on the compound (before he ever discovered its psychoactive properties) indicated that LSD has a pronounced effect upon the mammalian uterus. Sandoz's testing showed that LSD can stimulate uterine contractions with efficacy comparable to ergobasine, the active uterotonic component of the ergot fungus (Hofmann's work on ergot derivatives also produced a modified form of ergobasine which became a widely accepted medication used in obstetrics, under the trade name Methergine). Therefore, LSD use by pregnant women could be dangerous and is contraindicated.[1]
Initial studies in the 1960s and 70s raised concerns that LSD might produce genetic damage or developmental abnormalities in fetuses. However, these initial reports were based on in vitro studies or were poorly controlled and have not been substantiated. In studies of chromosomal changes in human users and in monkeys, the balance of evidence suggests no significant increase in chromosomal damage. For example, studies were conducted with people who had been given LSD in a clinical setting.[51] White blood cells from these people were examined for visible chromosomal abnormalities. Overall, there appeared to be no lasting changes. Several studies have been conducted using illicit LSD users and provide a less clear picture. Interpretation of these data is generally complicated by factors such as the unknown chemical composition of street LSD, concurrent use of other psychoactive drugs, and diseases such as hepatitis in the sampled populations. It seems possible that the small number of genetic abnormalities reported in users of street LSD is either coincidental or related to factors other than a toxic effect of pure LSD.[51]
Flashbacks and HPPD[edit | edit source]
"Flashbacks" are a reported psychological phenomenon in which an individual experiences an episode of some of LSD's subjective effects long after the drug has worn off — sometimes weeks, months, or even years afterward. Flashbacks can incorporate both positive and negative aspects of LSD trips. Flashbacks have proven difficult to study and are no longer officially recognized as a psychiatric syndrome. However, colloquial usage of the term persists and usually refers to any drug-free experience reminiscent of psychedelic drug effects, with the typical connotation that the episodes are of short duration.
No definitive explanation is currently available for these experiences. Any attempt at explanation must reflect several observations: first, over 70 percent of LSD users claim never to have "flashed back"; second, the phenomenon does appear linked with LSD use, though a causal connection has not been established; and third, a higher proportion of psychiatric patients report flashbacks than other users.[52] Several studies have tried to determine how likely a user of LSD, not suffering from known psychiatric conditions, is to experience flashbacks. The larger studies include Blumenfeld's in 1971[53] and Naditch and Fenwick's in 1977,[54] which arrived at figures of 20% and 28%, respectively.
Although flashbacks are not recognized as a medical syndrome, there is a potentially related syndrome in which LSD-like visual changes are persistent and cause clinically significant impairment or distress. This syndrome is called Hallucinogen Persisting Perception Disorder (HPPD), though not truly hallucinogenic, a DSM-IV diagnosis. Several scientific journal articles have described the disorder.[55] HPPD differs from flashbacks in that it is persistent and apparently entirely visual (although mood and anxiety disorders are sometimes diagnosed in the same individuals).
A recent review suggests that HPPD (as defined in the DSM-IV) is rare and affects only a distinctly vulnerable subpopulation of users.[56] However, it is possible that the prevalence of HPPD is underestimated because it can only be diagnosed in a person who admits to their health care practitioner that they have used psychotropics.[57]
There is no consensus regarding the nature and causes of HPPD (or flashbacks). Given that some symptoms have environmental triggers, it may represent a failure to adjust visual processing to changing environmental conditions. There are no explanations for why only some individuals develop HPPD. Explanations in terms of LSD physically remaining in the body for months or years after consumption have been discounted by experimental evidence.[52] Some say HPPD is a manifestation of post-traumatic stress disorder, not related to the direct action of LSD on brain chemistry, and varies according to the susceptibility of the individual to the disorder. Many emotionally intense experiences can lead to flashbacks when a person is reminded acutely of the original experience. However, not all published case reports of HPPD appear to describe an anxious hyper-vigilant state reminiscent of post-traumatic stress disorder. Instead, some cases appear to involve only visual symptoms.[52]
Psychosis[edit | edit source]
There are some cases of LSD inducing a psychosis in people who appeared to be healthy prior to taking LSD. This issue was reviewed extensively in a 1984 publication by Rick Strassman.[58] In most cases, the psychosis-like reaction is of short duration, but in other cases it may be chronic. It is difficult to determine if LSD itself induces these reactions or if it triggers latent conditions that would have manifested themselves otherwise. The similarities of time course and outcomes between putatively LSD-precipitated and other psychoses suggests that the two types of syndromes are not different and that LSD may have been a nonspecific trigger. Several studies have tried to estimate the prevalence of LSD-induced prolonged psychosis arriving at numbers of around 4 in 1,000 individuals (0.8 in 1,000 volunteers and 1.8 in 1,000 psychotherapy patients in Cohen 1960;[59] 9 per 1,000 psychotherapy patients in Melleson 1971).[60]
Chemistry[edit | edit source]
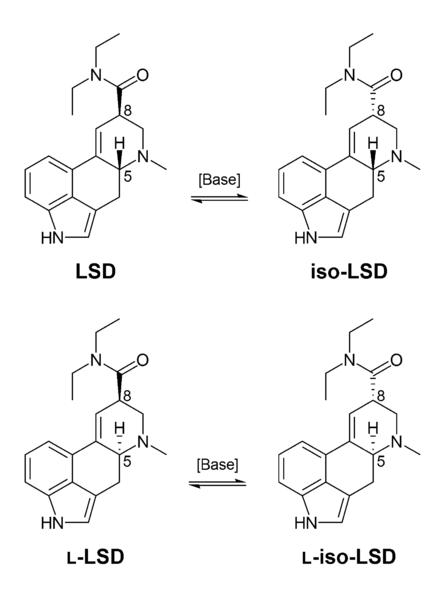
LSD is an ergoline derivative. It is commonly produced from reacting diethylamine with an activated form of lysergic acid. Activating reagents include phosphoryl chloride[61] and peptide coupling reagents.[62] Lysergic acid is made by alkaline hydrolysis of lysergamides like ergotamine, a substance derived from the ergot fungus on rye, or, theoretically, from ergine (lysergic acid amide, LSA), a compound that is found in morning glory (Ipomoea tricolor) and hawaiian baby woodrose (Argyreia nervosa) seeds. LSD is a chiral compound with two stereocenters at the carbon atoms C-5 and C-8, so that theoretically four different optical isomers of LSD could exist. LSD, also called (+)-D-LSD, has the absolute configuration (5R,8R). The C-5 isomers of lysergamides do not exist in nature and are not formed during the synthesis from D-lysergic acid. However, LSD and iso-LSD, the two C-8 isomers, rapidly interconvert in the presence of base. Non-psychoactive iso-LSD which has formed during the synthesis can be removed by chromatography and can be isomerized to LSD. A totally pure salt of LSD will emit small flashes of white light when shaken in the dark.[63] LSD is strongly fluorescent and will glow bluish-white under UV light.
Stability[edit | edit source]
"LSD," writes the chemist Alexander Shulgin, "is an unusually fragile molecule."[2] It is stable for indefinite amounts of time if stored, as a solid salt or dissolved in water, at low temperature and protected from air and light exposure. Two portions of its molecular structure are particularly sensitive, the carboxamide attachment at the 8-position and the double bond between the 8-position and the aromatic ring. The former is affected by high pH, and if perturbed will produce isolysergic acid diethylamide (iso-LSD), which is biologically inactive. If water or alcohol adds to the double bond (especially in the presence of light), LSD converts to "lumi-LSD", which is totally inactive in human beings, to the best of current knowledge. Furthermore, chlorine destroys LSD molecules on contact; even though chlorinated tap water typically contains only a slight amount of chlorine, because a typical LSD solution only contains a small amount of LSD, dissolving LSD in tap water is likely to completely eliminate the substance.[2]
A controlled study was undertaken to determine the stability of LSD in pooled urine samples.[64] The concentrations of LSD in urine samples were followed over time at various temperatures, in different types of storage containers, at various exposures to different wavelengths of light, and at varying pH values. These studies demonstrated no significant loss in LSD concentration at 25 °C for up to 4 weeks. After 4 weeks of incubation, a 30% loss in LSD concentration at 37 °C and up to a 40% at 45 °C were observed. Urine fortified with LSD and stored in amber glass or nontransparent polyethylene containers showed no change in concentration under any light conditions. Stability of LSD in transparent containers under light was dependent on the distance between the light source and the samples, the wavelength of light, exposure time, and the intensity of light. After prolonged exposure to heat in alkaline pH conditions, 10 to 15% of the parent LSD epimerized to iso-LSD. Under acidic conditions, less than 5% of the LSD was converted to iso-LSD. It was also demonstrated that trace amounts of metal ions in buffer or urine could catalyze the decomposition of LSD and that this process can be avoided by the addition of EDTA.
See also[edit | edit source]
- Mind at Large
- ALD-52
- Bogle-Chandler case, deaths mistakenly attributed to LSD overdoses
- Urban legends about LSD
- Entheogen
- Psychedelic psychotherapy
- United States v. Stanley, US Supreme Court case
- Related chemical compounds: ergolines, LSA, psilocybin, DMT, serotonin
- The 60s
- Methysergide and Sumatriptan - Prescription drugs closely related to LSD which treats migraine headaches.
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 Hofmann, Albert. LSD—My Problem Child (McGraw-Hill, 1980). ISBN 0-07-029325-2.
- ↑ 2.0 2.1 2.2 2.3 2.4 Alexander and Ann Shulgin. "LSD", in TiHKAL (Berkeley: Transform Press, 1997). ISBN 0-963-00969-9.
- ↑ "LSD: cultural revolution and medical advances". Royal Society of Chemistr. Retrieved 2007-09-27.
- ↑ "The Albert Hofmann Foundation". Hofmann Foundation. Retrieved 2007-09-27.
- ↑ Dr. Albert Hofmann[1]; translated from the original German (LSD Ganz Persönlich) by J. Ott. MAPS-Volume 6 Number 3 Summer 1996
- ↑ InfoFacts - LSD
- ↑ 7.0 7.1 Nichols, David (2003-05-24). "Hypothesis on Albert Hofmann's Famous 1943 "Bicycle Day"". Hofmann Foundation. Retrieved 2007-09-27.
- ↑ Hofmann, Albert (2003-05-24). "LSD: My Problem Child: Reflections on Sacred Drugs, Mysticism, and Science". Council on Spiritual Practices. Retrieved 2007-09-27.
- ↑ 9.0 9.1 Hofmann, Albert. "History Of LSD". Retrieved 2007-09-27.
- ↑ 10.0 10.1 10.2 Grof, Stanislav. LSD Psychotherapy. ISBN 978-0966001945.
- ↑ 11.0 11.1 11.2 11.3 "Chapter 3: Supreme Court Dissents Invoke the Nuremberg Code: CIA and DOD Human Subjects Research Scandals". Human Radiation Experiments. United States Department of Energy. Retrieved 2007-10-04.
- ↑ D. Moreno, Jonathan (September 1999). "Lessons Learned A Half-Century of Experimenting on Humans - U.S. Army experiments". Humanist. Retrieved 2007-10-04.
- ↑ Shane, Scott (2004-09-12). "Son probes strange death of WMD worker He believes agents murdered employee of Army to protect government secrets". Baltimore Sun. Retrieved 2007-10-04.
- ↑ Rob Evans, "MI6 pays out over secret LSD mind control tests". The Guardian 24 February 2006.
- ↑ The Lancet, Reviving research into psychedelic drugs 2006 367(9518):1214 [2]
- ↑ 16.0 16.1 Greiner T, Burch NR, Edelberg R (1958). "Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum". AMA Arch Neurol Psychiatry. 79 (2): 208–10. PMID 13497365.
- ↑ Stoll, W.A. (1947). Ein neues, in sehr kleinen Mengen wirsames Phantastikum. Schweiz. Arch. Neur. 60,483.
- ↑ Henderson, Leigh A.; Glass, William J. (1994). LSD: Still with us after all these years. ISBN 978-0787943790. Text " Jossey-Bass Inc., San Francisco " ignored (help)
- ↑ "LSD Vault: Dosage". 2006-07-06. Retrieved 2007-01-31. Check date values in:
|date=(help) - ↑ "LSD Toxicity: A Suspected Cause of Death". Journal of the Kentucky Medical Association. Retrieved 2007-11-26.
- ↑ Wolbach AB Jr, Isbell H, Miner EJ (1962). "Cross tolerance between mescaline and LSD-25, with a comparison of the mescaline and LSD reactions". Psychopharmacologia. 3: 1–14. doi:10.1007/BF00413101. PMID 14007904.
- ↑ Isbell H, Wolbach AB, Wikler A, Miner EJ (1961). "Cross Tolerance between LSD and Psilocybin". Psychopharmacologia. 2: E353. doi:10.1007/BF00407974. PMID 13717955.
- ↑ Huxley, Aldous The Doors of Perception and Heaven & Hell, Harper & Row, 1954
- ↑ Aghajanian, George K. and Bing, Oscar H. L. (1964). "Persistence of lysergic acid diethylamide in the plasma of human subjects" (PDF). Clin. Pharmacol. Ther. 5: 611–4. PMID 14209776.
- ↑ Papac DI, Foltz RL (1990). "Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry". J Anal Toxicol. 14 (3): 189–90. PMID 2374410.
- ↑ 26.0 26.1 Nichols, David E. (2004). "Psychotropics". Pharmacology & Therapeutics. 101 (2): 131–81. PMID 14761703.
- ↑ BilZ0r. "The Neuropharmacology of Hallucinogens: a technical overview". Erowid, v3.1 (August 2005).
- ↑ Svenningsson P. , Nairn A. C., Greengard P. (2005). "DARPP-32 Mediates the Actions of Multiple Drugs of Abuse". AAPS Journal. 07 (02): E353–E360. doi:10.1208/aapsj070235.
- ↑ Jacobs B. L., Heym J., Rasmussen K. (1983). "Raphe neurons: firing rate correlates with size of drug response". European Journal of Pharmacology. 90 (2–3): 275–8. doi:10.1016/0014-2999(83)90249-2. PMID 6873185.
- ↑ Gilberti, F. and Gregoretti, L. L. (1955). "Prime esperienze di antaonismo psicofarmacologico" (PDF). Sistema Nervoso. 4: 301–309.
- ↑ Agnew N, and Hoffer A. L. (1955). "Nicotinic acid modified lysergic acid diethylamide psychosis". J. Ment. Sci. 101: 12.
- ↑ Schiff PL (2006). "Ergot and its alkaloids". American journal of pharmaceutical education. 70 (5): 98. PMID 17149427.
- ↑ Kast, Eric (1967). "Attenuation of anticipation: a therapeutic use of lysergic acid diethylamide" (PDF). Psychiat. Quart. 41 (4): 646–57. doi:10.1007/BF01575629. PMID 4169685.
- ↑ Dr. Goadsby is quoted in "Research into psilocybin and LSD as cluster headache treatment", and he makes an equivalent statement in an Health Report interview on Australian Radio National (9 August 1999). Pages accessed 2007-01-31.
- ↑ Sewell, R. A. (2006-06-27). "Response of cluster headache to psilocybin and LSD". Neurology. 66 (12): 1920–2. doi:10.1212/01.wnl.0000219761.05466.43. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help);|access-date=requires|url=(help) - ↑ Summarized from "Research into psilocybin and LSD as cluster headache treatment" and the Clusterbusters website. Pages accessed 2007-01-31.
- ↑ The Good Drugs Guide. "LSD psychedelic effects". Retrieved 2008-03-03.
- ↑ 38.0 38.1 Linton, Harriet B. and Langs, Robert J. "Subjective Reactions to Lysergic Acid Diethylamide (LSD-25)". Arch. Gen. Psychiat. Vol. 6 (1962): 352–68.
- ↑ Cohen, S. (1959). The therapeutic potential of LSD-25. A Pharmacologic Approach to the Study of the Mind, p251–258.
- ↑ Chwelos N, Blewett D.B., Smith C.M., Hoffer A. (1959). "Use of d-lysergic acid diethylamide in the treatment of alcoholism". Quart. J. Stud. Alcohol. 20: 577–90. PMID 13810249.
- ↑ Maclean, J.R.; Macdonald, D.C.; Ogden, F.; Wilby, E., "LSD-25 and mescaline as therapeutic adjuvants." In: Abramson, H., Ed., The Use of LSD in Psychotherapy and Alcoholism, Bobbs-Merrill: New York, 1967, pp. 407–426; Ditman, K.S.; Bailey, J.J., "Evaluating LSD as a psychotherapeutic agent," pp.74–80; Hoffer, A., "A program for the treatment of alcoholism: LSD, malvaria, and nicotinic acid," pp. 353–402.
- ↑ Minogue, S. J. "Alcoholics Anonymous." The Medical Journal of Australia May 8 (1948):586–587.
- ↑ Mangini M (1998). "Treatment of alcoholism using psychedelic drugs: a review of the program of research". J Psychoactive Drugs. 30 (4): 381–418. PMID 9924844.
- ↑ Katz MM, Waskow IE, Olsson J (1968). "Characterizing the psychological state produced by LSD". J Abnorm Psychol. 73 (1): 1–14. doi:10.1037/h0020114. PMID 5639999.
- ↑ See, e.g., Gerald Oster's article "Moiré patterns and visual hallucinations". Psychedelic Rev. No. 7 (1966): 33–40.
- ↑ Grof, Stanislav (1979). Realms of the Human Unconcious (Observations from LSD Research). London: Souvenir Press (E & A) Ltd. pp. 13–14. ISBN 0 285 64882 9. Unknown parameter
|coauthors=ignored (help) - ↑ Video of the experiment can be viewed here.
- ↑ Pahnke, Walter N., Drugs and Mysticism: An Analysis of the Relationship between Psychedelic Drugs and the Mystical Consciousness. A thesis presented to the Committee on Higher Degrees in History and Philosophy of Religion, Harvard University, June 1963. Cited in Masters, R.E.L., & Houston, Jean., The Varieties of Psychedelic Experience (Turnstone Books, 1973).
- ↑ 49.0 49.1 "LSD and Antidepressants" (2003) via Erowid.
- ↑ Kit Bonson, "The Interactions between Hallucinogens and Antidepressants" (2006).
- ↑ 51.0 51.1 Dishotsky NI, Loughman WD, Mogar RE, Lipscomb WR (1971). "LSD and genetic damage" (PDF). Science. 172 (982): 431–40. doi:10.1126/science.172.3982.431. PMID.
- ↑ 52.0 52.1 52.2 David Abrahart (1998). "A Critical Review of Theories and Research Concerning Lysergic Acid Diethylamide (LSD) and Mental Health". Retrieved 2007-02-02.
- ↑ Blumenfield M (1971). "Flashback phenomena in basic trainees who enter the US Air Force". Military Medicine. 136 (1): 39–41. PMID 5005369.
- ↑ Naditch MP, Fenwick S (1977). "LSD flashbacks and ego functioning". Journal of Abnormal Psychology. 86 (4): 352–9. doi:10.1037/0021-843X.86.4.352. PMID 757972.
- ↑ See, for example, Abraham HD, Aldridge AM (1993). "Adverse consequences of lysergic acid diethylamide". Addiction. 88 (10): 1327–34. PMID 8251869.
- ↑ Halpern JH, Pope HG Jr (2003). "Hallucinogen persisting perception disorder: what do we know after 50 years?". Drug Alcohol Depend. 69 (2): 109–19. doi:10.1016/S0376-8716(02)00306-X. PMID 12609692.; Halpern JH (2003). "Hallucinogens: an update". Curr Psychiatry Rep. 5 (5): 347–54. doi:10.1007/s11920-003-0067-4. PMID 13678554. [3]
- ↑ Baggott et al. (2006) Prevalence of chronic flashbacks in hallucinogen users: a web-based questionnaire
- ↑ Strassman RJ (1984). "Adverse reactions to psychedelic drugs. A review of the literature". J Nerv Ment Dis. 172 (10): 577–95. PMID 6384428.
- ↑ Cohen, Sidney (1960). "Lysergic Acid Diethylamide: Side Effects and Complications" (PDF). Journal of Nervous and Mental Disease. 130 (1): 30–40. PMID 13811003. Unknown parameter
|month=ignored (help) - ↑ Malleson, Nicholas (1971). "Acute Adverse Reactions to LSD in Clinical and Experimental Use in the United Kingdom" (PDF). Brit. J. Psychiat. 118 (543): 229–30. PMID 4995932.
- ↑ Aaron P. Monte, Danuta Marona-Lewicka, Arthi Kanthasamy, Elaine Sanders-Bush, and David E. Nichols. (1995). "Stereoselective LSD-like Activity in a Series of D-Lysergic Acid Amides of (R)- and (S)-2-Aminoalkanes". J. Med. Chem. 38 (6): 958–966. doi:10.1021/jm00006a015.
- ↑ Nichols, D. E.; Frescas, S.; Marona-Lewicka, D.; Kurrasch-Orbaugh, D. M. (2002). "Lysergamides of Isomeric 2,4-Dimethylazetidines Map the Binding Orientation of the Diethylamide Moiety in the Potent Hallucinogenic Agent N,N-Diethyllysergamide (LSD)". J. Med. Chem. 45 (19): 4344–4349. doi:10.1021/jm020153s.
- ↑ "Erowid Online Books : "TIHKAL" - #26 LSD-25". Retrieved 2007-11-12.
- ↑ Li Z., McNally A. J., Wang H., Salamone S. J. (1998). "Stability study of LSD under various storage conditions". J. Anal. Toxicol. 22 (6): 520–5. PMID 9788528. Unknown parameter
|month=ignored (help)
Further reading[edit | edit source]
- Stevens, Jay. Storming Heaven: LSD And The American Dream ([6])
- Marks, John. The Search for the Manchurian Candidate: The CIA and Mind Control (1979), 0-8129-0773-6
- Grof, Stanislav. LSD Psychotherapy. (April 10, 2001)
- Lee, Martin A. and Bruce Shlain. Acid Dreams: The Complete Social History of LSD: The CIA, the Sixties, and Beyond [7]
External links[edit | edit source]
| Wikinews has related news: Drug website surveys LSD users and culture |
- Erowid -> LSD Information
- The Lycaeum Archive -> LSD
- TIHKAL entry for LSD
- The Neurochemistry of Psychedelic Experience, Science & Consciousness Review
- Current LSD Research – MAPS
- International 3 day conference on LSD in Basel
- Talk to FrankUK government anti-drug site on LSD
- Scholarly bibliography on the histories of LSD use
Template:Hallucinogenic lysergamides
Template:Link FA Template:Link FA
af:LSD als:LSD bs:LSD bg:ЛСД ca:LSD cs:Diethylamid kyseliny lysergové da:LSD de:LSD et:LSD el:Διαιθυλαμίδιο του λυσεργικού οξέος eo:LSD eu:LSD fa:الاسدی fo:LSD gl:LSD hr:Lisergična dietilamidna kiselina it:LSD he:LSD ka:ლსდ lv:Lizergīnskābes dietilamīds lt:LSD hu:LSD nl:Lyserginezuurdiëthylamide no:Lysergsyredietylamid nn:LSD ksh:LysergSäureDiäthylamid scn:LSD simple:Lysergic acid diethylamide sk:Dietylamid kyseliny lysergovej sl:LSD sr:ЛСД sh:LSD fi:LSD sv:LSD uk:ЛСД
 KSF
KSF