Macrolide
 From Wikidoc - Reading time: 3 min
From Wikidoc - Reading time: 3 min
|
WikiDoc Resources for Macrolide |
|
Articles |
|---|
|
Most recent articles on Macrolide |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Macrolide at Clinical Trials.gov Clinical Trials on Macrolide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Macrolide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Macrolide Discussion groups on Macrolide Directions to Hospitals Treating Macrolide Risk calculators and risk factors for Macrolide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Macrolide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
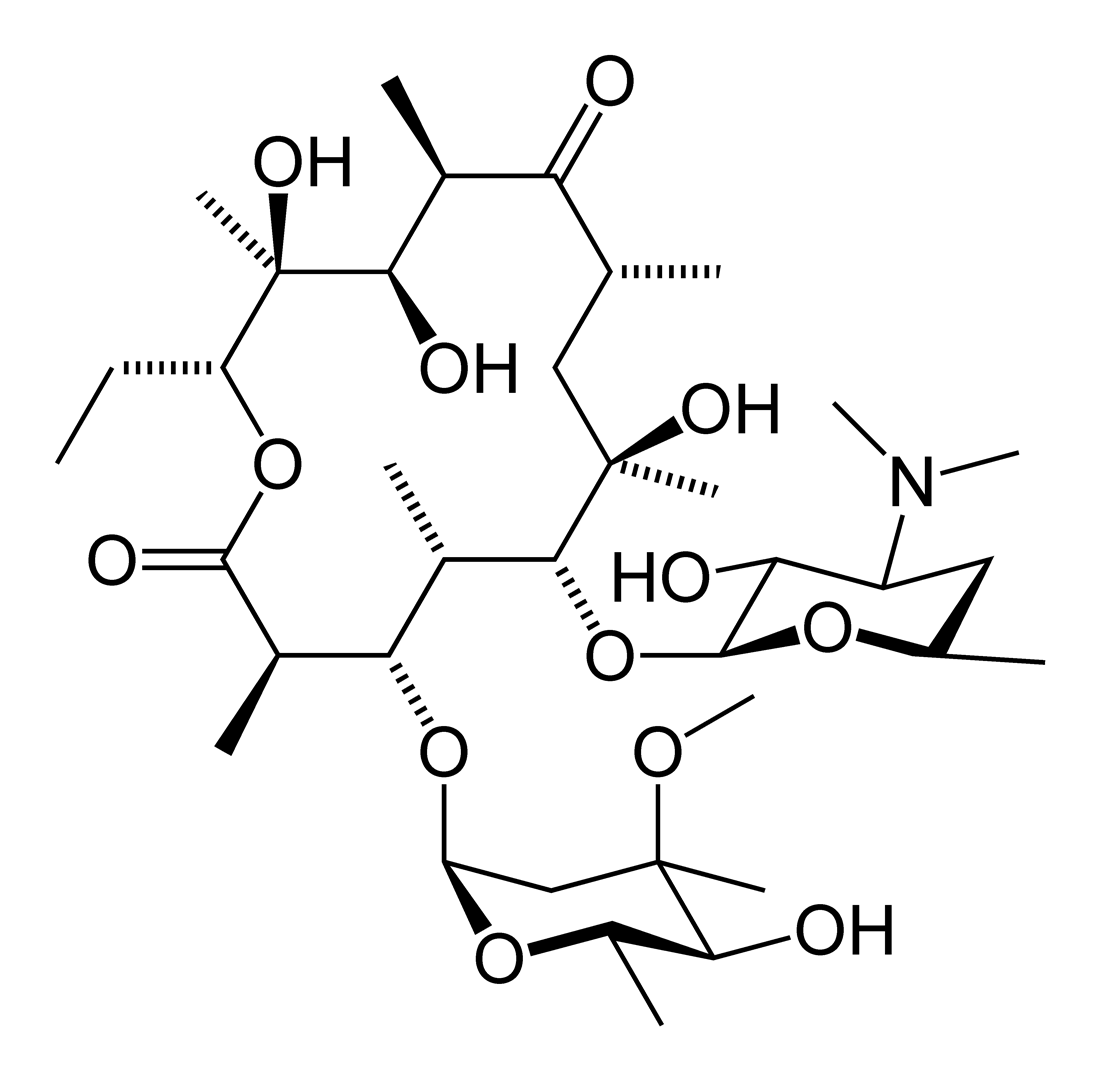
Overview[edit | edit source]
The macrolides are a group of drugs (typically antibiotics) whose activity stems from the presence of a macrolide ring, a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14, 15 or 16-membered. Macrolides belong to the polyketide class of natural products.
Members[edit | edit source]
Common antibiotic macrolides[edit | edit source]
- Azithromycin (Zithromax, Zitromax, Sumamed) - Unique, does not inhibit CYP3A4
- Clarithromycin (Biaxin)
- Dirithromycin (Dynabac)
- Erythromycin
- Roxithromycin (Rulid, Surlid,Roxid)
Developmental macrolides[edit | edit source]
- Carbomycin A
- Josamycin
- Kitasamycin
- Midecamicine/midecamicine acetate
- Oleandomycin
- Spiramycin
- Troleandomycin
- Tylosin/tylocine (Tylan)
Ketolides[edit | edit source]
Ketolides are a new class of antibiotics that are structurally related to the macrolides. They are used to fight respiratory tract infections caused by macrolide-resistant bacteria.
- Telithromycin (Ketek)
- Cethromycin
Others include spiramycin (used for treating toxoplasmosis), ansamycin, oleandomycin, carbomycin and tylocine.
Non-antibiotic macrolides[edit | edit source]
The drug tacrolimus (Prograf), which is used as an immunosuppressant, is also a macrolide. It has similar activity to cyclosporin.
Toxic macrolides[edit | edit source]
A variety of toxic macrolides produced by bacteria have been isolated and characterized, such as the mycolactones.
Uses[edit | edit source]
Antibiotic macrolides are used to treat infections such as respiratory tract and soft tissue infections. The antimicrobial spectrum of macrolides is slightly wider than that of penicillin, and therefore macrolides are a common substitute for patients with a penicillin allergy. Beta-hemolytic streptococci, pneumococci, staphylococci and enterococci are usually susceptible to macrolides. Unlike penicillin, macrolides have been shown to be effective against mycoplasma, mycobacteria, some rickettsia and chlamydia.
Mechanism of action[edit | edit source]
The mechanism of action of the macrolides is inhibition of bacterial protein biosynthesis by binding reversibly to the subunit 50S of the bacterial ribosome, thereby inhibiting translocation of peptidyl tRNA. This action is mainly bacteriostatic, but can also be bactericidal in high concentrations. Macrolides tend to accumulate within leukocytes, and are therefore actually transported into the site of infection.
A novel, non-antibiotic, anti-inflammatory effect of 14-membered macrolides was discovered in Japan (Kudo S. et al.), which is especially effective in improving control of diffuse panbronchiolitis (DPB). Research on the anti-inflammatory properties of the macrolide ring is ongoing.
Resistance[edit | edit source]
The primary means of bacterial resistance to macrolides occurs by post-transcriptional methylation of the 23S bacterial ribosomal RNA. This acquired resistance can be either plasmid-mediated or chromosomal, i.e through mutation, and results in cross-resistance to macrolides, lincosamides, and streptogramins (an MLS-resistant phenotype).
Two other types of acquired resistance rarely seen include the production of drug-inactivating enzymes (esterases or kinases) as well as the production of active ATP-dependent efflux proteins that transport the drug outside of the cell.
Side effects[edit | edit source]
A recent British Medical Journal article highlights that the combination of macrolides and statins (used for lowering cholesterol) is not advisable and can lead to debilitating myopathy. This is because macrolides are potent inhibitors of the cytochrome P450 system, particularly of CYP3A4. Macrolides also have a class effect of QT prolongation. Macrolides exhibit enterohepatic recycling; that is, the drug is absorbed in the gut and sent to the liver, only to be excreted into the duodenum in bile from the [[liver]. This can lead to a build up of the product in the system causing nausea.
References[edit | edit source]
- Macrolide Antibiotics: Chemistry, Biology, and Practice, 2nd Edition, Ed. Satoshi Omura, 2002, Academic Press.
External links[edit | edit source]
ar:ماكرولايد
de:Makrolide
fo:Makrolid
it:Macrolide
nl:Macrolide antibioticum
fi:Makrolidi
sv:Makrolid
 KSF
KSF