Meropenem clinical pharmacology
 From Wikidoc - Reading time: 3 min
From Wikidoc - Reading time: 3 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Clinical Pharmacology[edit | edit source]
Pharmacokinetics[edit | edit source]
Plasma Concentrations[edit | edit source]
At the end of a 30-minute intravenous infusion of a single dose of MERREM I.V. in normal volunteers, mean peak plasma concentrations are approximately 23 µg/mL (range 14-26) for the 500 mg dose and 49 µg/mL (range 39-58) for the 1 g dose. A 5-minute intravenous bolus injection of MERREM I.V. in normal volunteers results in mean peak plasma concentrations of approximately 45 µg/mL (range 18-65) for the 500 mg dose and 112 µg/mL (range 83-140) for the 1 g dose.
Following intravenous doses of 500 mg, mean plasma concentrations of meropenem usually decline to approximately 1 µg/mL at 6 hours after administration.
No accumulation of meropenem in plasma or urine was observed with regimens using 500 mg administered every 8 hours or 1 g administered every 6 hours in volunteers with normal renal function.
Distribution[edit | edit source]
The plasma protein binding of meropenem is approximately 2%.
Meropenem penetrates well into most body fluids and tissues including cerebrospinal fluid, achieving concentrations matching or exceeding those required to inhibit most susceptible bacteria. After a single intravenous dose of MERREM I.V., the highest mean concentrations of meropenem were found in tissues and fluids at 1 hour (0.5 to 1.5 hours) after the start of infusion, except where indicated in the tissues and fluids listed in the table below.
Meropenem Concentrations in Selected Tissues (Highest Concentrations Reported)
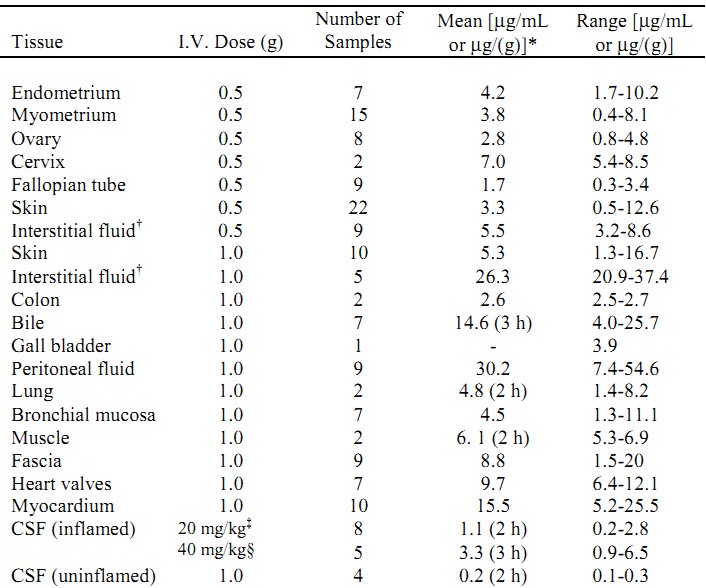 |
* at 1 hour unless otherwise noted
† obtained from blister fluid
‡ in pediatric patients of age 5 months to 8 years
§ in pediatric patients of age 1 month to 15 years
Metabolism[edit | edit source]
There is one metabolite that is microbiologically inactive.
Excretion[edit | edit source]
In subjects with normal renal function, the elimination half-life of MERREM I.V. is approximately 1 hour. Approximately 70% of the intravenously administered dose is recovered as unchanged meropenem in the urine over 12 hours, after which little further urinary excretion is detectable. A further 28% is recovered as the microbiologically inactive metabolite. Fecal elimination represents only approximately 2% of the dose. The measured renal clearance and the effect of probenecid show that meropenem undergoes both filtration and tubular secretion.
Urinary concentrations of meropenem in excess of 10 µg/mL are maintained for up to 5 hours after a 500 mg dose.
Speicific Populations[edit | edit source]
Renal Impairment[edit | edit source]
Pharmacokinetic studies with MERREM I.V. in patients with renal insufficiency have shown that the plasma clearance of meropenem correlates with creatinine clearance. Dosage adjustments are necessary in subjects with renal impairment. (See DOSAGE AND ADMINISTRATION - Use in Adults with Renal Impairment.) A pharmacokinetic study with MERREM I.V. in elderly patients with renal insufficiency has shown a reduction in plasma clearance of meropenem that correlates with age-associated reduction in creatinine clearance.
Meropenem I.V. is hemodialyzable. However, there is no information on the usefulness of hemodialysis to treat overdosage.
Hepatic Impairment[edit | edit source]
A pharmacokinetic study with MERREM I.V. in patients with hepatic impairment has shown no effects of liver disease on the pharmacokinetics of meropenem.
Geriatric Patients[edit | edit source]
A pharmacokinetic study with MERREM I.V. in elderly patients with renal impairment showed a reduction in plasma clearance of meropenem that correlates with age-associated reduction in creatinine clearance.
Pediatric Patients[edit | edit source]
The pharmacokinetics of MERREM I.V. in pediatric patients 2 years of age or older are essentially similar to those in adults. The elimination half-life for meropenem was approximately 1.5 hours in pediatric patients of age 3 months to 2 years. The pharmacokinetics are linear over the dose range from 10 to 40 mg/kg.
Drug Interactions[edit | edit source]
Probenecid competes with meropenem for active tubular secretion and thus inhibits the renal excretion of meropenem. Following administration of probenecid with meropenem, the mean systemic exposure increased 56% and the mean elimination half-life increased 38%. Co-administration of probenecid with meropenem is not recommended.[1]
References[edit | edit source]
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050706s022lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.
 KSF
KSF