Messenger RNA
 From Wikidoc - Reading time: 8 min
From Wikidoc - Reading time: 8 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
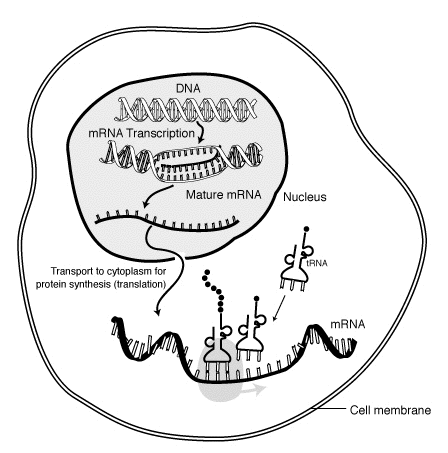
Messenger Ribonucleic Acid (mRNA) is a molecule of RNA encoding a chemical "blueprint" for a protein product. mRNA is transcribed from a DNA template, and carries coding information to the sites of protein synthesis: the ribosomes. Here, the nucleic acid polymer is translated into a polymer of amino acids: a protein. In mRNA as in DNA, genetic information is encoded in the sequence of four nucleotides arranged into codons of three bases each. Each codon encodes for a specific amino acid, except the stop codons that terminate protein synthesis. This process requires two other types of RNA: Transfer RNA (tRNA) mediates recognition of the codon and provides the corresponding amino acid, while Ribosomal RNA (rRNA) is the central component of the ribosome's protein manufacturing machinery.
mRNA "life cycle"[edit | edit source]
The brief life of an mRNA molecule begins with transcription and ultimately ends in degradation. During its life, an mRNA molecule may also be processed, edited, and transported prior to translation. Eukaryotic mRNA molecules often require extensive processing and transport, while prokaryotic molecules do not.
Transcription[edit | edit source]
During transcription, RNA polymerase makes a copy of a gene from the DNA to mRNA as needed. This process is similar in eukaryotes and prokaryotes. One notable difference, however, is that eukaryotic RNA polymerase associates with mRNA processing enzymes during transcription so that processing can proceed quickly after the start of transcription. The short-lived, unprocessed or partially processed, product is termed pre-mRNA; once completely processed, it is termed mature mRNA.
Eukaryotic pre-mRNA processing[edit | edit source]
Processing of mRNA differs greatly among eukaryotes, bacteria and archea. Non-eukaryotic mRNA is essentially mature upon transcription and requires no processing, except in rare cases. Eukaryotic pre-mRNA, however, requires extensive processing.
5' cap addition[edit | edit source]
A 5' cap (also termed an RNA cap, an RNA 7-methylguanosine cap or an RNA m7G cap) is a modified guanine nucleotide that has been added to the "front" or 5' end of a eukaryotic messenger RNA shortly after the start of transcription. The 5' cap consists of a terminal 7-methylguanosine residue which is linked through a 5'-5'-triphosphate bond to the first transcribed nucleotide. Its presence is critical for recognition by the ribosome and protection from RNases.
Cap addition is coupled to transcription, and occurs co-transcriptionally, such that each influences the other. Shortly after the start of transcription, the 5' end of the mRNA being synthesized is bound by a cap-synthesizing complex associated with RNA polymerase. This enzymatic complex catalyzes the chemical reactions that are required for mRNA capping. Synthesis proceeds as a multi-step biochemical reaction.
Splicing[edit | edit source]
Splicing is the process by which pre-mRNA is modified to remove certain stretches of non-coding sequences called introns; the stretches that remain include protein-coding sequences and are called exons. Sometimes pre-mRNA messages may be spliced in several different ways, allowing a single gene to encode multiple proteins. This process is called alternative splicing. Splicing is usually performed by an RNA-protein complex called the spliceosome, but some RNA molecules are also capable of catalyzing their own splicing (see ribozymes).
Editing[edit | edit source]
In some instances, an mRNA will be edited, changing the nucleotide composition of that mRNA. An example in humans is the apolipoprotein B mRNA, which is edited in some tissues, but not others. The editing creates an early stop codon, which upon translation, produces a shorter protein.
Polyadenylation[edit | edit source]
Polyadenylation is the covalent linkage of a polyadenylyl moiety to a messenger RNA molecule. In eukaryotic organisms, most messenger RNA (mRNA) molecules are polyadenylated at the 3' end. The poly(A) tail and the protein bound to it aid in protecting mRNA from degradation by exonucleases. Polyadenylation is also important for transcription termination, export of the mRNA from the nucleus, and translation. mRNA can also be polyadenylated in prokaryotic organisms, where poly(A) tails act to facilitate, rather than impede, exonucleolytic degradation.
Polyadenylation occurs during and immediately after transcription of DNA into RNA. After transcription has been terminated, the mRNA chain is cleaved through the action of an endonuclease complex associated with RNA polymerase. The cleavage site is characterized by the presence of the base sequence AAUAAA near the cleavage site. After the mRNA has been cleaved, 80 to 250 adenosine residues are added to the free 3' end at the cleavage site. This reaction is catalyzed by polyadenylate polymerase.
Transport[edit | edit source]
Another difference between eukaryotes and prokaryotes is mRNA transport. Because eukaryotic transcription and translation is compartmentally separated, eukaryotic mRNAs must be exported from the nucleus to the cytoplasm. Mature mRNAs are recognized by their processed modifications and then exported through the nuclear pore.
Translation[edit | edit source]
Because prokaryotic mRNA does not need to be processed or transported, translation by the ribosome can begin immediately after the end of transcription. Therefore, it can be said that prokaryotic translation is coupled to transcription and occurs co-transcriptionally.
Eukaryotic mRNA that has been processed and transported to the cytoplasm (i.e. mature mRNA) can then be translated by the ribosome. Translation may occur at ribosomes free-floating in the cytoplasm, or directed to the endoplasmic reticulum by the signal recognition particle. Therefore, unlike prokaryotes, eukaryotic translation is not directly coupled to transcription.
Degradation[edit | edit source]
After a certain amount of time, the message is degraded by RNases into its component nucleotides. The limited longevity of mRNA enables a cell to alter protein synthesis rapidly in response to its changing needs.
Different mRNAs within the same cell have distinct lifetimes. In bacterial cells, individual mRNAs can survive from seconds to more than an hour; in mammalian cells, mRNA lifetimes range from several minutes to days. The greater the stability of an mRNA, the more protein may be produced from that transcript. The presence of AU-rich motifs in some mammalian mRNAs tends to destabilize those transcripts through the action of cellular proteins that bind these motifs. Rapid mRNA degradation via AU-rich motifs is a critical mechanism for preventing the overproduction of potent cytokines such as tumor necrosis factor (TNF) and granulocyte-macrophage colony stimulating factor (GM-CSF).[1][verification needed] Base pairing with a small interfering RNA (siRNA) or microRNA (miRNA) can also accelerate mRNA degradation.
mRNA structure[edit | edit source]

5' cap[edit | edit source]
The 5' cap is a modified guanine nucleotide added to the "front" (5' end) of the pre-mRNA using a 5',5-Triphosphate linkage. This modification is critical for recognition and proper attachment of mRNA to the ribosome, as well as protection from 5' exonucleases. It may also be important for other essential processes, such as splicing and transport.
Coding regions[edit | edit source]
Coding regions are composed of codons, which are decoded and translated into one (mostly eukaryotes) or several (mostly prokaryotes) proteins by the ribosome. Coding regions begin with the start codon and end with the one of three possible stop codons. In addition to protein-coding, portions of coding regions may also serve as regulatory sequences in the pre-mRNA as exonic splicing enhancers or exonic splicing silencers. Start codons are indicated by a AUG triplet. Stop codons are indicated by a UAA, UAG, or UGA.
Untranslated regions[edit | edit source]
Untranslated regions (UTRs) are sections of the RNA before the start codon and after the stop codon that are not translated, termed the five prime untranslated region (5' UTR) and three prime untranslated region (3' UTR), respectively. These regions are transcribed as part of the same transcript as the coding region. Several roles in gene expression have been attributed to the untranslated regions, including mRNA stability, mRNA localization, and translational efficiency. The ability of a UTR to perform these functions depends on the sequence of the UTR and can differ between mRNAs.
The stability of mRNAs may be controlled by the 5' UTR and/or 3' UTR due to varying affinity for RNA degrading enzymes called ribonucleases and for ancillary proteins that can promote or inhibit RNA degradation.
Translational efficiency, including sometimes the complete inhibition of translation, can be controlled by UTRs. Proteins that bind to either the 3' or 5' UTR may affect translation by influencing the ribosome's ability to bind to the mRNA. MicroRNAs bound to the 3' UTR also may affect translational efficiency or mRNA stability.
Cytoplasmic localization of mRNA is thought to be a function of the 3' UTR. Proteins that are needed in a particular region of the cell can actually be translated there; in such a case, the 3' UTR may contain sequences that allow the transcript to be localized to this region for translation.
Some of the elements contained in untranslated regions form a characteristic secondary structure when transcribed into RNA. These structural mRNA elements are involved in regulating the mRNA. Some, such as the SECIS element, are targets for proteins to bind. One class of mRNA element, the riboswitches, directly bind small molecules, changing their fold to modify levels of transcription or translation. In these cases, the mRNA regulates itself.
3' poly(A) tail[edit | edit source]
The 3' poly(A) tail is a long sequence of adenine nucleotides (often several hundred) added to the "tail" or 3' end of the pre-mRNA through the action of an enzyme, polyadenylate polymerase. In higher eukaryotes, the poly(A) tail is added onto transcripts that contain a specific sequence, the AAUAAA signal. The importance of the AAUAAA signal is demonstrated by a mutation in the human alpha 2-globin gene that changes the original sequence AATAAA into AATAAG, which can lead to hemoglobin deficiencies.[2]
Monocistronic versus polycistronic mRNA[edit | edit source]
An mRNA molecule is said to be monocistronic when it contains the genetic information to translate only a single protein. This is the case for most of the eukaryotic mRNAs[3]. On the other hand, polycistronic mRNA carries the information of several proteins, which are translated into several proteins. Most of the mRNA found in bacteria and archea are polycistronic[3]. Dicistronic is the term used to describe a mRNA that encodes only two proteins.
Anti-sense mRNA[edit | edit source]
During transcription, double stranded DNA produces mRNA from the sense strand; the other, complementary, strand of DNA is termed anti-sense. Anti-sense mRNA is an RNA complementary in sequence to one or more mRNAs. In some organisms, the presence of an anti-sense mRNA can inhibit gene expression by base-pairing with the specific mRNAs. In biochemical research, this effect has been used to study gene function, by simply shutting down the studied gene by adding its anti-sense mRNA transcript. Such studies have been done on the worm Caenorhabditis elegans and the bacterium Escherichia coli. This plays a part in RNA interference and RNA transcription.
See also[edit | edit source]
References[edit | edit source]
- ↑ Shaw, G. and Kamen, R. "A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation." Cell. 1986 Aug 29;46(5):659-67. PMID: 3488815 CELL
- ↑ Higgs DR, Goodbourn SE, Lamb J, Clegg JB, Weatherall DJ, Proudfoot NJ. (1983). "α-thalassaemia caused by a polyadenylation signal mutation". Nature. 306 (5941): 398–400. PMID 6646217 doi:10.1038/306398a0.
- ↑ 3.0 3.1
Kozak, M. (1983). "Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles" (PDF). Microbiological Reviews. 47 (1): 1–45. PMID 6343825. Retrieved 2006-08-12. Unknown parameter
|month=ignored (help)
External links[edit | edit source]
- Life of mRNA Flash animation
ar:مرسال الحمض النووي الريبي bg:Информационна РНК ca:ARN missatger cs:MRNA da:MRNA de:MRNA et:Informatsiooni-RNA id:MRNA it:RNA messaggero he:MRNA la:MRNA lt:IRNR nl:Messenger RNA sk:Mediátorová ribonukleová kyselina sv:Budbärar-RNA uk:Матрична рибонуклеїнова кислота ur:پیامبر آر این اے
 KSF
KSF