Monoclonal antibodies
 From Wikidoc - Reading time: 7 min
From Wikidoc - Reading time: 7 min
Overview[edit | edit source]
Monoclonal antibodies (mAb or moAb) are antibodies that are identical because they are produced by one type of immune cell that are all clones of a single parent cell. Given (almost) any substance, it is possible to create monoclonal antibodies that specifically bind to that substance; they can then serve to detect or purify that substance. This has become an important tool in biochemistry, molecular biology and medicine.
When used as medications, the generic name ends in -mab (see "Nomenclature of monoclonal antibodies").
Discovery[edit | edit source]
The idea of a "magic bullet" was first proposed by Paul Ehrlich who at the beginning of the 20th century postulated that if a compound could be made that selectively targeted a disease-causing organism, then a toxin for that organism could be delivered along with the agent of selectivity.
In the 1970s the B-cell cancer myeloma was known, and it was understood that these cancerous B-cells all produce a single type of antibody (a paraprotein). This was used to study the structure of antibodies, but it was not yet possible to produce identical antibodies specific to a given antigen.
A process of producing monoclonal antibodies involving human-mouse hybrid cells was described by Jerrold Schwaber in 1973[1] and remains widely cited among those using human-derived hybridomas.[2], but claims to priority have been controversial. A science history paper on the subject gave some credit to Schwaber for inventing a technique that was widely cited, but stopped short of suggesting that he had been cheated[3]. The invention is generally accredited to Georges Köhler, César Milstein, and Niels Kaj Jerne in 1975;[4] who shared the Nobel Prize in Physiology or Medicine in 1984 for the discovery. The key idea was to use a line of myeloma cells that had lost their ability to secrete antibodies, come up with a technique to fuse these cells with healthy antibody producing B-cells, and be able to select for the successfully fused cells.
In 1988 Greg Winter and his team pioneered the techniques to humanize monoclonal antibodies,[5] removing the reactions that many monoclonal antibodies caused in some patients.
Production[edit | edit source]




Hybridoma Cell Production[edit | edit source]
Monoclonal antibodies are made by fusing the spleen cells from a mouse that has been immunized with the desired antigen with myeloma cells.
Polyethylene glycol is used to fuse of adjacent plasma membranes, but the success rate is low so a selective medium is used in which only fused cells can grow. This is because myeloma cells that have lost the ability to synthesize hypoxanthine-guanine-phosphoribosyl transferase (HGPRT).

This enzyme enables cells to synthesize purines using an extracellular source of hypoxanthine as a precursor ordinarily, the absence of HGPRT is not a problem for the cell because cells have an alternate biochemical pathway that they can use to synthesize purines. However, when cells are exposed to aminopterin (a folic acid analogue), they are unable to use this other, rescue, pathway and are now fully dependent on HGPRT for survival.
The selective culture medium is called HAT medium because it contains:
• Hypoxanthine
• Aminopterin
• Thymidine
This medium is selective for fused, (hydridoma) cells because unfused myeloma cells cannot grow because they lack HGPRT. Unfused normal spleen cells cannot grow indefinitely because of their limited life span. However, hybridoma cells are able to grow indefinitely because the spleen cell partner supplies HGPRT and the myeloma partner is immortal because it is a cancer cell.
The fused hybrid cells are called hybridomas, and since they are derived from cancer cells, are immortal and can be grown idefinitely.
This mixture of cells is then diluted and clones are grown from single parent cells. The antibodies secreted by the different clones are then tested for their ability to bind to the antigen (for example with a test such as EIA or Antigen Microarray Assay) or immuno-dot blot, and the most productive and stable clone is then grown in culture medium to a high volume. When the hybridoma cells are injected in mice (in the peritoneal cavity, the gut), they produce tumors containing an antibody-rich fluid called ascites fluid.
The medium must be enriched during selection to further favour hybridoma growth. This can be achieved by the use of a layer of feeder fibrocyte cells or supplement medium such as briclone. Production in cell culture is usually preferred as the ascites technique is painful to the animal and if replacement techniques exist, is considered unethical.
Recombinant[edit | edit source]
The production of Recombinant monoclonal antibodies involves technologies, referred to as repertoire cloning or phage display/yeast display. Recombinant antibody engineering involves the use of viruses or yeast to create antibodies, rather than mice. These techniques rely on rapid cloning of immunoglobulin gene segments to create libraries of antibodies with slightly different amino acid sequences from which antibodies with desired specificities can be selected.[6] These techniques can be used to enhance: the specificity with which antibodies recognize antigens, their stability in various environmental conditions, their therapeutic efficacy, and their detectability in diagnostic applications.[7] Fermentation chambers have been used to produce these antibodies on a large scale.
Applications[edit | edit source]
Once monoclonal antibodies for a given substance have been produced, they can be used to detect the presence and quantity of this substance, for instance in a Western blot test (to detect a protein on a membrane) or an immunofluorescence test (to detect a substance in a cell). They are also very useful in immunohistochemistry which detect antigen in fixed tissue sections. Monoclonal antibodies can also be used to purify a substance with techniques called immunoprecipitation and affinity chromatography.
Monoclonal antibodies for cancer treatment[edit | edit source]
One possible treatment for cancer involves monoclonal antibodies that bind only to cancer cell-specific antigens and induce an immunological response against the target cancer cell. Such mAb could also be modified for delivery of a toxin, radioisotope, cytokine or other active conjugate; it is also possible to design bispecific antibodies that can bind with their Fab regions both to target antigen and to a conjugate or effector cell. In fact, every intact antibody can bind to cell receptors or other proteins with its Fc region. The illustration below shows all these possibilities:
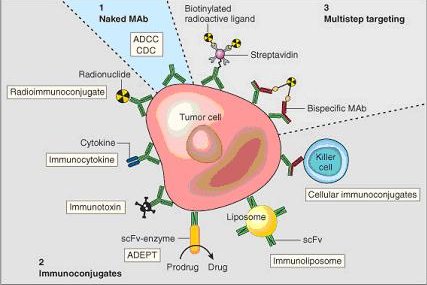
Chimeric and humanized antibodies[edit | edit source]
One problem in medical applications is that the standard procedure of producing monoclonal antibodies yields mouse antibodies. Although murine antibodies are very similar to human ones there are differences. The human immune system hence recognizes mouse antibodies as foreign, rapidly removing them from circulation and causing systemic inflammatory effects.
A solution to this problem would be to generate human antibodies directly from humans. However, this is not easy, primarily because it is generally not seen as ethical to challenge humans with antigen in order to produce antibody; while the ethics of doing the same to non-humans is a matter of debate. Furthermore, it is not easy to generate human antibodies against human tissues.
Various approaches using recombinant DNA technology to overcome this problem have been tried since the late 1980s. In one approach, one takes the DNA that encodes the binding portion of monoclonal mouse antibodies and merges it with human antibody producing DNA. One then uses mammalian cell cultures to express this DNA and produce these half-mouse and half-human antibodies. (Bacteria cannot be used for this purpose, since they cannot produce this kind of glycoprotein.) Depending on how big a part of the mouse antibody is used, one talks about chimeric antibodies or humanized antibodies. Another approach involves mice genetically engineered to produce more human-like antibodies. Monoclonal antibodies have been generated and approved to treat: cancer, cardiovascular disease, inflammatory diseases, macular degeneration, transplant rejection, multiple sclerosis, and viral infection (see monoclonal antibody therapy).
In August 2006 the Pharmaceutical Research and Manufacturers of America reported that U.S. companies had 160 different monoclonal antibodies in clinical trials or awaiting approval by the Food and Drug Administration.[9]
See also[edit | edit source]
References[edit | edit source]
- ↑ Schwaber, J and Cohen, E. P., "Human x Mouse Somatic Cell Hybrid Clones Secreting Immunoglobulins of Both Parental Types," Nature, 244 (1973), 444--447.
- ↑ Science Citation Index
- ↑ Alberto Cambrosio Peter Keating, Journal of the History of Biology. Between fact and technique: The beginnings of hybridoma technology, Volume 25,Issue 2,175- 230.[1]
- ↑ Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975;256:495-7. PMID 1172191. Reproduced in J Immunol 2005;174:2453-5. PMID 15728446.
- ↑ Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature 1988;332:323-7. PMID 3127726.
- ↑ Siegel DL (2002). "Recombinant monoclonal antibody technology". Transfusion clinique et biologique : journal de la Société française de transfusion sanguine. 9 (1): 15–22. PMID 11889896.
- ↑ Schmitz U, Versmold A, Kaufmann P, Frank HG (2000). "Phage display: a molecular tool for the generation of antibodies--a review". Placenta. 21 Suppl A: S106–12. PMID 10831134.
- ↑ Modified from Carter P: Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer 2001;1:118-129
- ↑ PhRMA Reports Identifies More than 400 Biotech Drugs in Development. Pharmaceutical Technology, August 24, 2006. Retrieved 2006-09-04.
External links[edit | edit source]
- Monoclonal Antibodies, from John W. Kimball's online biology textbook
- Monoclonal+antibodies at the US National Library of Medicine Medical Subject Headings (MeSH)
de:Monoklonaler Antikörper it:Anticorpi monoclonali he:נוגדנים חד-שבטיים sl:Monoklonska protitelesa sv:Monoklonal antikropp
 KSF
KSF