NMDA receptor antagonist
 From Wikidoc - Reading time: 8 min
From Wikidoc - Reading time: 8 min
Overview[edit | edit source]
NMDA receptor antagonists are a class of anesthetics that work to antagonize, or inhibit the action of, the N-methyl d-aspartate receptor (NMDAR). They are used as anesthesia for animals and, less commonly, for humans; the state of anesthesia they induce is referred to as dissociative anesthesia. However, there is evidence that NMDA receptor antagonists can cause a certain type of brain damage referred to as Olney's Lesions (in rodents).
Some NMDA receptor antagonists, such as ketamine and phencyclidine (PCP), are popular as recreational drugs for their hallucinogenic properties. When used recreationally, they are classified as dissociative drugs. Because some users use them for spiritual reasons, these recreational NMDA receptor antagonists are sometimes considered entheogens.
Uses and effects[edit | edit source]
NMDA receptor antagonists induce a state called dissociative anesthesia, which is marked by catalepsy, amnesia, and analgesia.[1] Ketamine and other NMDA receptor antagonists are most frequently used in conjunction with diazepam as anesthesia in cosmetic or reconstructive plastic surgery[2] and in the treatment of burn victims.[3] Ketamine is a favored anesthetic for emergency patients with unknown medical history because it depresses breathing and circulation less than other anesthetics.[4] The NMDA receptor antagonist dextromethorphan is one of the most commonly used cough suppressants in the world.[5]
Depressed NMDA receptor function is associated with an array of negative symptoms. For example, NMDA receptor hypofunction that occurs as the brain ages may be partially responsible for memory deficits associated with aging.[6] Schizophrenia may also have to do with inadequate NMDA receptor function (the "glutamate hypothesis" of schizophrenia).[7] NMDA receptor antagonists can mimic these problems; they sometimes induce "psychotomimetic" side effects, symptoms resembling psychosis. Such side effects caused by NMDA receptor inhibitors include hallucinations, paranoid delusions, confusion, difficulty concentrating, agitation, alterations in mood, nightmares,[8] catatonia,[9] ataxia,[10] anaesthesia,[11] and learning and memory deficits.[12]
Because of these psychotomimetic effects, NMDA receptor antagonists, especially phencyclidine, ketamine, and dextromethorphan, are used as recreational drugs. At subanesthetic doses, these drugs have mild stimulant effects, and at higher doses, begin inducing dissociation and hallucinations.[13]
Most NMDA receptor antagonists are metabolized in the liver.[14][15] Frequent administration of most NMDA receptor antagonists can lead to tolerance, whereby the liver will more quickly eliminate NMDA receptor antagonists from the bloodstream.[16]
Neurotoxicity[edit | edit source]
Exposure to NMDA receptor antagonists may cause a serious brain damage in the cingulate cortex and retrosplinial cortex regions of the brain. The experimental NMDA receptor antagonist MK-801 has been shown to cause neural vacuolization in test rodents that later develop into irreversible lesions called "Olney's Lesions."[17][18] Many drugs have been found that lessen the risk of neurotoxicity from NMDA receptor antagonists. Centrally acting alpha 2 agonists such as clonidine and guanfacine are thought to most specifically target the etiology of NMDA neurotoxicity. Other drugs acting on various neurotransmitter systems known to inhibit NMDA antagonist neurotoxicity include: anticholinergics, diazepam, barbiturates,[19] ethanol,[20] 5-HT2A serotonin agonists,[21] and muscimol.[22]
Potential for treatment of excitotoxicity[edit | edit source]
Since NMDA receptors are one of the most harmful factors in excitotoxicity, antagonists of the receptors have held much promise for the treatment of conditions that involve excitotoxicity, including traumatic brain injury, stroke, and neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's. However, because of the neurotoxicity caused by NMDA receptor antagonists, research has slowed[23] and studies have started to find agents that prevent this neurotoxicity.[22][20] Most clinical trials involving NMDA receptor antagonists have failed due to unwanted side effects of the drugs; since the receptors also play an important role in normal glutamatergic function, blocking them has harmful effects.[24] This interference with normal function could be responsible for neuronal death that sometimes results from NMDA receptor antagonist use.[25]
Mechanism of action[edit | edit source]
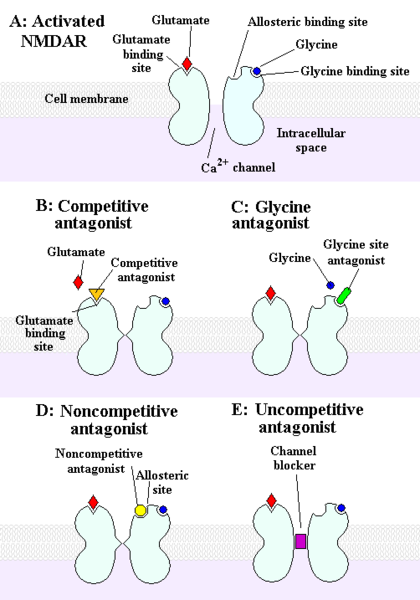
The NMDA receptor is an ionotropic receptor that allows for the transfer of electrical signals between neurons in the brain and in the spinal column. For electrical signals to pass, the NMDA receptor must be open. To remain open, an NMDA receptor must bind to glutamate and to glycine. An NMDA receptor that is bound to glycine and glutamate and has an open ion channel is called "activated."
The receptor can be deactivated by inhibitors that can cause the NMDAR to close by binding to allosteric sites. Chemicals that deactivate the NMDA receptor are called antagonists. NMDAR antagonists fall into four categories: Competitive antagonists, which bind to and block the binding site of the neurotransmitter glutamate; glycine antagonists, which bind to and block the glycine site; noncompetitive antagonists, which inhibit NMDARs by binding to allosteric sites; and uncompetitive antagonists, which block the ion channel by binding to a site within it.[10]
Examples[edit | edit source]
Uncompetitive channel blockers include:
- Amantadine – used for treating Parkinson's disease and influenza.[26][27]
- Dextromethorphan – a common antitussive found in cough medicines.[28]
- Dextrorphan – a Schedule I controlled substance in the United States.[28][29]
- Dizocilpine (MK-801) – an experimental drug.[30]
- Ibogaine – a Schedule I controlled substance in the United States.[31][29]
- Ketamine – an animal and human anesthetic and recreational drug.[32]
- Nitrous oxide – used for anesthesia, particularly in dentistry.[33]
- Phencyclidine, a Schedule II controlled substance in the United States.[29]
- Riluzole – used to treat amyotrophic lateral sclerosis.[34]
- Tiletamine – an animal anesthetic.[35]
Noncompetitive antagonists include:
- Aptiganel (Cerestat, CNS-1102) – binds the Mg2+ binding site within the channel of the NMDA receptor.
- Memantine (Axura®, Akatinol®, Namenda®, Ebixa®, 1-amino-3,5-dimethylada-mantane) – moderate affinity, voltage-dependent uncompetitive antagonist.[36] Approved in the U.S. by the Food and Drug Administration for the treatment of Alzheimer's disease.[37]
- Remacimide – principle metabolite is an uncompetitive antagonist with a low affinity for the binding site.[38]
Glycine antagonists (drugs that act at the glycine binding site) include:
- 7-chlorokynurenate[39]
- DCKA (5,7-dichlorokynurenic)[40]
Competitive antagonists include:
- AP7 (2-amino-7-phosphonoheptanoic acid)[41]
- APV (R-2-amino-5-phosphonopentanoate)[42]
- CPPene (3-[(R)-2-carboxypiperazin-4-yl]-prop-2-enyl-1-phosphonic acid)[43]
See also[edit | edit source]
- Neurotransmitters
- Psychedelics
- Long-term potentiation
- NMDA
- AMPA
- AMPA receptor
- Calcium/calmodulin-dependent protein kinases
References[edit | edit source]
- ↑ Pender J (1971). "Dissociative anesthesia". JAMA. 215 (7): 1126–30. PMID 5107596.
- ↑ Ersek R (2004). "Dissociative anesthesia for safety's sake: ketamine and diazepam--a 35-year personal experience". Plast Reconstr Surg. 113 (7): 1955–9. PMID 15253183.
- ↑ Ceber M, Salihoglu T. "Ketamine may be the first choice for anesthesia in burn patients". J Burn Care Res. 27 (5): 760–2. PMID 16998413.
- ↑ Heshmati F, Zeinali M, Noroozinia H, Abbacivash R, Mahoori A (2003). "Use of ketamine in severe status asthmaticus in intensive care unit". Iran J Allergy Asthma Immunol. 2 (4): 175–80. PMID 17301376.
- ↑ Equinozzi R, Robuschi M (2006). "Comparative Efficacy and Tolerability of Pholcodine and Dextromethorphan in the Management of Patients with Acute, Non-Productive Cough : A Randomized, Double-Blind, Multicenter Study". Treat Respir Med. 5 (6): 509–513. PMID 17154678.
- ↑ Newcomer, JW (2001). "NMDA receptor regulation of memory and behavior in humans". Hippocampus. 11 (5): 529–542. PMID 11732706. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ Lipina, T (2005). "Modulators of the glycine site on NMDA receptors, D-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia". Psychopharmacology. 179 (1): 54–67. PMID 15759151. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ Muir, KW (1995). "Clinical experience with excitatory amino acid antagonist drugs". Stroke. 26 (3): 503–513. Retrieved 2007-01-17. Unknown parameter
|coauthors=ignored (help) - ↑ Aarts, MM (2003). "Novel treatment of excitotoxicity: targeted disruption of intracellular signalling from glutamate receptors". Biochemical Pharmacology. 66 (6): 877–886. PMID 12963474. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ 10.0 10.1 10.2 Kim AH, Kerchner GA, and Choi DW. (2002). "Blocking Excitotoxicity". In CNS Neuroproteciton. Marcoux FW and Choi DW, editors. Springer, New York. Pages 3-36.
- ↑ Kristensen, JD (1992). "The NMDA-receptor antagonist CPP abolishes neurogenic 'wind-up pain' after intrathecal administration in humans". Pain. 51 (2): 249–253. PMID 1484720. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ Rockstroh, S (1996). "Effects of the novel NMDA-receptor antagonist SDZ EAA 494 on memory and attention in humans". Psychopharmacology. 124 (3): 261–266. PMID 8740048. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ Lim D (2003). "Ketamine associated psychedelic effects and dependence". Singapore Med J. 44 (1): 31–4. PMID 12762561.
- ↑ Chia YY, Liu K, Chow LH, Lee TY (1999). "The preoperative administration of intravenous dextromethorphan reduces postoperative morphine consumption". Anesth. Analg. 89 (3): 748–52. PMID 10475318.
- ↑ Kharasch ED, Labroo R (1992). "Metabolism of ketamine stereoisomers by human liver microsomes". Anesthesiology. 77 (6): 1201–7. PMID 1466470.
- ↑ Livingston A, Waterman AE (1978). "The development of tolerance to ketamine in rats and the significance of hepatic metabolism". Br. J. Pharmacol. 64 (1): 63–9. PMID 698482.
- ↑ Olney J, Labruyere J, Price M (1989). "Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs". Science. 244 (4910): 1360–2. PMID 2660263.
- ↑ Hargreaves R, Hill R, Iversen L. "Neuroprotective NMDA antagonists: the controversy over their potential for adverse effects on cortical neuronal morphology". Acta Neurochir Suppl (Wien). 60: 15–9. PMID 7976530.
- ↑ Olney J, Labruyere J, Wang G, Wozniak D, Price M, Sesma M (1991). "NMDA antagonist neurotoxicity: mechanism and prevention". Science. 254 (5037): 1515–8. PMID 1835799.
- ↑ 20.0 20.1 Farber, NB (2004). "In the adult CNS, ethanol prevents rather than produces NMDA antagonist-induced neurotoxicity". PMID 15518643. Retrieved 2007-01-18.
- ↑ Farber N, Hanslick J, Kirby C, McWilliams L, Olney J (1998). "Serotonergic agents that activate 5HT2A receptors prevent NMDA antagonist neurotoxicity". Neuropsychopharmacology. 18 (1): 57–62. PMID 9408919.
- ↑ 22.0 22.1 Farber, NB (2003). "Muscimol prevents NMDA antagonist neurotoxicity by activating GABAA receptors in several brain regions". PMID 14642834. Retrieved 2007-01-18.
- ↑ Maas, AI (2001). "Neuroprotective agents in traumatic brain injury". Expert Opinion On Investigational Drugs. 10 (4): 753–767. PMID 11281824. Retrieved 2007-01-17.
- ↑ Chen, HS. "The chemical biology of clinically tolerated NMDA receptor antagonists". Journal of Neurochemistry. 97 (6): 1611–1126. PMID 16805772. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ Gardoni, F (2006). "New targets for pharmacological intervention in the glutamatergic synapse". European Journal of Pharmacology. 545 (1): 2–10. PMID 16831414. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ "Effects of N-Methyl-D-Aspartate (NMDA)-Receptor Antagonism on Hyperalgesia, Opioid Use, and Pain After Radical Prostatectomy", University Health Network, Toronto, September 2005
- ↑ "MedlinePlus Drug Information: Amantadine." MedlinePlus website Accessed May 29, 2007
- ↑ 28.0 28.1 Wong BY, Coulter DA, Choi DW, Prince DA (1988). "Dextrorphan and dextromethorphan, common antitussives, are antiepileptic and antagonize N-methyl-D-aspartate in brain slices". Neurosci. Lett. 85 (2): 261–6. PMID 2897648.
- ↑ 29.0 29.1 29.2 Controlled Substances Act. Accessed from the US Drug Enforcement Administration website on May 29, 2007.
- ↑ Fix AS, Horn JW, Wightman KA; et al. (1993). "Neuronal vacuolization and necrosis induced by the noncompetitive N-methyl-D-aspartate (NMDA) antagonist MK(+)801 (dizocilpine maleate): a light and electron microscopic evaluation of the rat retrosplenial cortex". Exp. Neurol. 123 (2): 204–15. doi:10.1006/exnr.1993.1153. PMID 8405286.
- ↑ Popik P, Layer RT, Skolnick P (1994): "The putative anti-addictive drug ibogaine is a competitive inhibitor of [3H]MK-801 binding to the NMDA receptor complex." Psychopharmacology (Berl), 114(4), 672-4. Abstract
- ↑ Harrison N, Simmonds M (1985). "Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex". Br J Pharmacol. 84 (2): 381–91. PMID 2858237.
- ↑ Grasshoff C, Drexler B, Rudolph U, Antkowiak B (2006). "Anaesthetic drugs: linking molecular actions to clinical effects". Curr. Pharm. Des. 12 (28): 3665–79. PMID 17073666.
- ↑ Hugon J (1996). "ALS therapy: targets for the future". Neurology. 47 (6 Suppl 4): S251–4. PMID 8959997.
- ↑ Ko JC, Smith TA, Kuo WC, Nicklin CF (1998). "Comparison of anesthetic and cardiorespiratory effects of diazepam-butorphanol-ketamine, acepromazine-butorphanol-ketamine, and xylazine-butorphanol-ketamine in ferrets". Journal of the American Animal Hospital Association. 34 (5): 407–16. PMID 9728472.
- ↑ Robinson, DM (2006). "Memantine: a review of its use in Alzheimer's disease". Drugs. 66 (11): 1515–1534. PMID 16906789. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ Chawla, PS (2006). "What's new in clinical pharmacology and therapeutics". WMJ. 105 (3): 24–29. PMID 16749321. Retrieved 2007-01-17. Unknown parameter
|coauthors=ignored (help) - ↑ Muir, KW (2005). "Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists". Current Opinion in Pharmacology. 6 (1): 53–60. PMID 16359918.
|access-date=requires|url=(help) - ↑ Hartley DM, Monyer H, Colamarino SA, Choi DW (1990). "7-Chlorokynurenate Blocks NMDA Receptor-Mediated Neurotoxicity in Murine Cortical Culture". Eur J Neurosci. 2 (4): 291–295. PMID 12106035.
- ↑ Frankiewicz T, Pilc A, Parsons C (2000). "Differential effects of NMDA-receptor antagonists on long-term potentiation and hypoxic/hypoglycaemic excitotoxicity in hippocampal slices". Neuropharmacology. 39 (4): 631–42. PMID 10728884.
- ↑ van den Bos R, Charria Ortiz G, Cools A (1992). "Injections of the NMDA-antagonist D-2-amino-7-phosphonoheptanoic acid (AP-7) into the nucleus accumbens of rats enhance switching between cue-directed behaviours in a swimming test procedure". Behav Brain Res. 48 (2): 165–70. PMID 1535501.
- ↑ Abizaid A, Liu Z, Andrews Z, Shanabrough M, Borok E, Elsworth J, Roth R, Sleeman M, Picciotto M, Tschöp M, Gao X, Horvath T (2006). "Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite". J Clin Invest. 116 (12): 3229–39. PMID 17060947.
- ↑ Eblen F, Löschmann P, Wüllner U, Turski L, Klockgether T (1996). "Effects of 7-nitroindazole, NG-nitro-L-arginine, and D-CPPene on harmaline-induced postural tremor, N-methyl-D-aspartate-induced seizures, and lisuride-induced rotations in rats with nigral 6-hydroxydopamine lesions". Eur J Pharmacol. 299 (1–3): 9–16. PMID 8901001.
 KSF
KSF