Neurofibromatosis type I
 From Wikidoc - Reading time: 17 min
From Wikidoc - Reading time: 17 min
| Neurofibromatosis type I | |
 | |
|---|---|
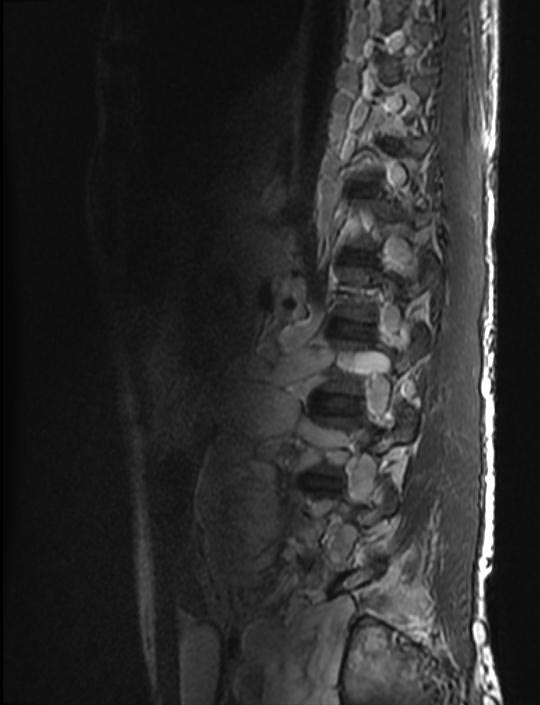
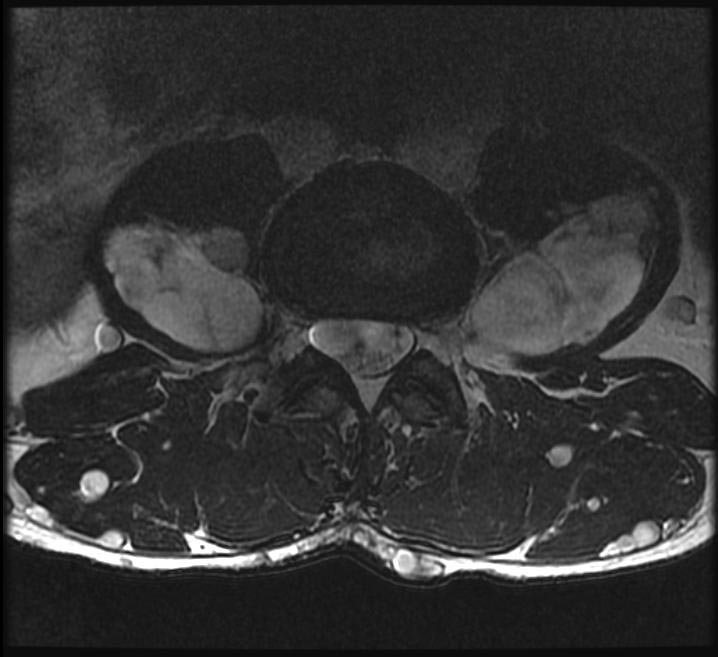
| This coronal contrast-enhanced fat-saturated T1 weighted image from an MRI of the pelvis shows the characteristic fasciculated appearance and infiltrative growth pattern of a plexiform neurofibroma. | |
| ICD-10 | Q85.0 |
| ICD-9 | 237.71 |
| OMIM | 162200 |
| DiseasesDB | 8937 |
| MedlinePlus | 000847 |
| eMedicine | derm/287 neuro/248 oph/338 radio/474 |
| MeSH | D009456 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Moises Romo, M.D.
Neurofibromatosis type I (NF-1), also known as von Recklinghausen syndrome, is a common inherited disease.
Along with neurofibromatosis type II (a.k.a. MISME syndrome), tuberous sclerosis, Sturge-Weber, and Von Hippel-Lindau disease, NF1 is a member of the phakomatoses or neurocutaneous syndromes, all of which have both neurologic and dermatologic lesions. This grouping is historical and based on disease pathology rather than genetic diagnosis.
Historical perspective[edit | edit source]
Overview[edit | edit source]
Neurofibromatosis type 1 was fully described in1882 by German pathologyst Friedrich von Recklinghausen, for which the disorder was eponymously named after him (von Recklinghausen disease), although the recognition of individuals with this phenotype probably dates back from the Hellenistic era.[1]
Discovery[edit | edit source]
- The first description of inviduals with Neurofibromatosis type 1 phenotype dates back from the XIV century, and probably even the Hellenistic era, made by Madigan, Schaw, and Masello, described as a "monstruous disease".[1][2][3]
- In 1785, English physician Mark Akensidi presents major reports about a patient, café au lait spots, rubbery lesions, and cognitive imapirment, and a big head, which he named "wart man".[4]
- In 1830, Prusian anatomist and physician, Theodor Schwann described the nerve sheath cover that later was appreciated as the predominant cell type in neurofibromatosis.[5]
- In 1847, Prusian pathologist, Rudolf Virchow stated that connective tissue tumors that contained nerves, should be classified apart from those that do not contain nerves, naming them "fibroma molluscum" or "elephantiasis molluscum".[6]
- In 1873, German ophtalmologist, Julius von Michel first reported the case of a patient with optic glioma.[4][7]
- In 1882, German pathologist, Friedrich von Recklinghause systemically described Neurofibromatosis type 1 with the presentation of two of his patients, recognizing it as a nosologic entity in his treatise "Über die multiplen Fibrome der Haut und ihre Beziehung zu den multiplen Neuromen".[1][8][5]
- In 1900, English physician A. Thomson describes Neurofibromatosis as an hereditary entity.[5][4]
- In 1909, Suzuki was the first to describe an association between pheochromocytoma and neurofibromatosis type 1.[5][4]
- In 1987, gene linkage studies localised the NF1 gene in chromosome 17.[9]
- In 1987 this condition was uniformly recognized and clasified thanks to the National Institutes of Health (NIH) Consensus Development Conference.[1]
- NF1 was cloned in 1990,[10] and its gene product neurofibromin was identified in 1992.[11]
- In 1999, Nielsen et al. discovered that loss of expression of p16 protein was the cause of malignant transformation in neurofibromatosis type 1.[4][12]
Cultural history of Neurofibromatosis type 1[edit | edit source]
- Probably the most famous case of neurofibromatosis type 1 was described in 1888, in England, with Joseph Merrick, the "elephant man". This man had a difficult life, were he was cruely exhibited in circuses as a phenomenon. Joseph was born as a healthy child in 1862, but started to develope typical characteristics of the disease at age 2.[13] By age 22, Merrick presented dysmorphic facies, a big head-size, skin and bone deformities, but surprisingly, no cognitive impairment. He quoted:
"The deformity I am exhibiting now is because an elephant scared my mother; she walked down the street as a procession of animals paraded. A huge crowd gathered to watch them, and unfortunately they pushed my mother under the feet of an elephant. She was very scared. She was pregnant with me, and this misfortune was the cause of my deformity".
"Is true that my form is something odd, but blaming me is blaming God; could I create myself anew would not fail pleasing you.If I could reach from pole to pole or grasp the ocean with a span, I would be measured by the soul. The mind´s the standard of the man.[14]
- This patient was the ispiration for the 1980 movie "The Elephant Man", by david Lynch.
Impact of Neurofibromatosis type 1[edit | edit source]
Society's acceptance and understanding for neurofibromatosis type 1 has changed drastically over the time; from beign a so-called a "montruous disease", to a well known entity now in day, compassion have grown for those individuals suffering from this condition thanks to the many research behind its behavior and characteristics. Advances in its treatment have made a great impact in the natural histoty of this disease, decreasing mortality and now focusing on a more multidysciplinary management.[6]
Famous cases of Neurofibromatosis type 1[edit | edit source]
Joseph Merrick (the elephant man)[15][14][13]
Munya Yassir (actress)[16]
Aaron Anderson (brother of actress Gillian Anderson)[17]
Nick Gilbert (son of Cleveland-Cavaliers owner, Dan Gilberet)[18]
Alexander Owens[18]
Pathophysiology[edit | edit source]
Overview[edit | edit source]
Normal physiology[edit | edit source]
Neurofibromin 1 (NF1) gene, is a large multi-domain protein, of approximately 250 KDa, containing 2,818 amino acids,[9][19] located in chromosome 17,[20] codes for the GTPase-activating protein, neurofibromin.[21][22] This protein, is a tumor suppressor expressed in many cells, primarily in neurons, glial, immune cells, edothelial cellls of the adrenal medulla, Schwann cells and early in melanocyte development.[23][24]
Neurofibromin works by downregulating the RAS/MAPK pathway, by accelerating hydrolisis and preventing cell proliferation.[22][23] [25][26]
Neurofibromin interacts cytoskeletal microtubular and microfilaments for proper neuronal development.[9][27]
Pathogenesis and genetics[edit | edit source]
Neurofibromatosis type 1 is an autosomal syndrome, although, roughly 50% of gene mutations arise from de novo events.[9]
The large size of the NF1 gene (350 kb with 60 exons) predisposes to a high mutation rate, with approximately one mutation occurring per 10,000 gametes.[28]
NF1 mutations lead to protein truncation (loss of neurofibromin expression), which in turn produces increased cell growth and survival through expression of RAS to ultimately increase chances for malignant transformation.[24][29][9]
More than 3,000 NF1 gene mutations have been recognised.[24]
Loss of neurofibromin leads to formation of skin lesions, tumours and skeletal abnormalities.[24]
Patients with neurofibromatosis type 1 syndrome have a higher incidence of GI, liver, lung, bone, thyroid, breast and ovarian cancers.[9][30]
Nerve sheath tumours can arise from benign neurofibromas and these compose the first cause of death in individuals with neurofibromatosis type 1.[9]
Patients with neurofibromatosis type 1 can present a wide variability of clinical features, even in the same family since mutation mechanisms are so vast. Interestingly, patients can present traits limited to a part of their body (mosaicism).[24]
Therapies are focused to inhibit RAS prenylation to block oncogenic RAS signalling.[9]
Classification[edit | edit source]
There is no classification for neurofibromatosis type 1.
Associated conditions[edit | edit source]
- Neurofibromatosis type 2 is a genetic condition which may be inherited or may arise spontaneously. It is caused by NF2 gene(Merlin gene) mutation in chromosome 22 and presents with bilateral vestibular schwannomas(acoustic neuromas).[31]
Gross pathology[edit | edit source]
The most common non-neoplastic manifestations are cafe-au-lait macules, which is a very sensitive finding when they appear in large quantities.[24][32][33]
Dysplasia of the long bones and sphenoid wings, fractures, and pseudoarthrosis are common findings in patients with neurofibromatosis type 1.[33][34] They occur due to osteoblastic and osteoclastic dysfunction which lead to decreased mineralization.[24]
The defects in bone observed in individuals with neurofibromatosis type 1 are due to the loss of both copies of NF1 in osteoclasts and/or osteoblasts.Osteoblast dysfunction following Nf1 loss results in an increased generation of pyrophosphate, which inhibits bone mineral (hydroxyapatite) production and bone mineralization, causing reduced bone density and a higher risk of bone fracture.[24][35]
Lisch nodules are hyperpigmented hamartomas of the iris, which are seen in 95–100% of adults withneurofibromatosis type 1.[33][34]
Neurofibromas are benign peripheral nerve sheath tumors that arise from Schwann cells, and represent the most frequent type of tumours in patients with neurofibromatosis type 1; they can be divided as dermal and plexiform neurofibromas.[24][36]
Optic gliomas are the most common brain tumors in neurofibromatosis type 1, they are observed in 15–20% of children younger than 7 years.[33][37]
Several types of cerebrovascular disease, including stenotic or occlusive disease, vascular dilatation or aneurysm, arteriovenous fistula, moyamoya syndrome, or vascular proliferation have been consistently found in biopsies of patients with neurofibromatosis type 1.[33][38]
Microscopic pathology[edit | edit source]
Cafe au-lait macules consist of a dense population of melanocytes with biallelic NF1 inactivation.[24][32]
Neurofibromas are benign peripheral nerve sheath tumors composed of neoplastic Schwann cells, fibroblasts, blood vessels, and mast cells.[33]
Optic pathway gliomas are composed of astrocytes, oligodendrocytes, neurons, microglia and progenitor or stem cells.[24][39]
Epidemiology and demographics[edit | edit source]
Neurofibromatosis type 1 is the most common single gene disorder in humans, occurring in about 4 in 100,000 births worldwide.[40]
Clinical findings[edit | edit source]
Peripheral nervous system lesions[edit | edit source]
Between birth and infancy : CALMs(cafe au lait macules) • Orbital dysplasia • Tibial dysplasia • Pseudarthrosis Plexiform neurofibroma[24]
Between infancy and early chidhood: Learning deficits • ADHD or ASD • Motor and/or speech delays Optic pathway glioma[24]
Between early childhood and adolescence: • Skinfold freckling • Lisch nodules, Scoliosis • Dermal neurofibroma • Paraspinal neurofibroma Brainstem glioma[24]
In adulthood: • MPNST • Breast cancer • High-grade glioma[24]
A neurofibroma is a mass lesion of the peripheral nervous system. Its cellular lineage is uncertain, and may derive from Schwann cells, other perineural cell lines, or fibroblasts. Neurofibromas may arise sporadically, or in association with NF-1. A neurofibroma may arise at any point along a peripheral nerve. A cutaneous neurofibroma manifests as a solitary or as multiple firm, rubbery bumps of varying sized on a person's skin. A solitary neurofibroma may also occur in a deeper nerve trunk, and only be seen on cross-sectional imaging (e.g. computed tomography or magnetic resonance) as a fusiform enlargement of a nerve.
The hallmark lesion of NF-1 is the plexiform neurofibroma. These lesions are composed of sheets of neurofibromatous tissue which may infiltrate and encase major nerves, blood vessels, and other vital structures. Because of this, these lesions are impossible to routinely resect.
If a plexiform fibroma manifests on a leg or arm, it will cause extra blood circulation, and may thus accelerate the growth of the limb. This may cause considerable difference in length between left and right limbs. To equalise the difference during childhood, there is an orthopedic surgery called "epiphysiodesis", in which the epiphyseal (growth) plate is removed. It can be removed in one of the bone's end to slow down the growth, or in both ends to stop growth of that bone completely. The surgery must also be carefully planned with regard to timing, as it is non-reversible, so that the limbs are at near equal length at end of growth.
Schwannomas are peripheral nerve-sheath tumor seen with increased frequency in NF-1. In practice, the major distinction between a schwannoma and a solitary neurofibroma is that a schwannoma can be resected while sparing the underlying nerve, while resection of a neurofibroma requires the sacrifice of the underlying nerve.
Malignant peripheral nerve-sheath tumors, or neurofibrosarcomas, can arise from degeneration of a plexiform neurofibroma; this is, fortunately, a rare complication.
Dermatologic manifestations[edit | edit source]
In addition to the cutaneous neurofibroma, patients with NF-1 develop flat pigmented lesions of the skin called café au lait spots.[2].
NF-1 patients may also get freckles of the axillae (armpits).
http://en.wikipedia.org/wiki/Plexiform_neurofibroma
Central nervous system manifestations[edit | edit source]
The primary neurologic involvement is of the peripheral nervous system, as described above.
Intracranially, NF-1 patients have a predisposition to develop glial tumors of the central nervous system; primarily: optic gliomas and astrocytomas. Another CNS manifestation of NF1 is the so-called "unidentified bright object" or UBO, which is a lesion which has increased signal on a T2 weighted sequence of a magnetic resonance imaging examination of the brain. These UBOs are typically found in the cerebellar peduncles, pons, midbrain, globus pallidus, thalamus, and optic radiations. Their exact identity remains a bit of a mystery since they disappear over time (usually, by age 16), and they are not typically biopsied or resected. They may represent a focally degenerative bit of myelin.
Within the CNS, this manifests as a weakness of the dura, which is the tough covering of the brain and spine. Weakness of the dura leads to focal enlargement (termed dural ectasia) due to chronic exposure to the pressures of CSF pulsation.
Radiographically, dural ectasia can lead to scalloping of the posterior vertebral bodies and to the formation of cystic diverticula of the dura of the spine (termed meningoceles).
Skeletal lesions[edit | edit source]
Bones, especially the ribs, can develop chronic erosions (pits) from the constant pressure of adjacent neurofibromas and schwannomas. Similarly, the neural foramen of the spine can be widened due to the presence of a nerve root neurofibroma or schwannoma.
In NF-1, these is also a generalized abnormality of the soft tissues, which is referred to as mesodermal dysplasia. This manifests as maldevelopment of skeletal structures, including
- Focal scoliosis and/or kyphosis, which is the most common skeletal manifestation of NF-1, occurring in 20% of affected patients. Approximately one quarter of patients will require corrective surgery.
- Bowing of a long bone with a tendency to fracture and not heal, yielding a pseudarthrosis. The most common bone to be affected is the tibia (causing congenital pseudarthrosis of the tibia or CPT). CPT occurs in 2-4% of individuals with NF-1.
- Malformation of the facial bones or of the eye sockets (lambdoid suture defects, sphenoid dysplasia)
- Unilateral overgrowth of a limb
Cognitive problems and learning disabilities in NF-1[edit | edit source]
The most common complication in patients with NF-1 is cognitive and learning disability. These cognitive problems have been shown to be present in approximately 80% of children with NF-1 and have significant effects on their schooling and everyday life.[41] The most common cognitive problems are with perception, executive functioning and attention. ADHD has been shown to be present in approximately 38% of children with NF-1. Language, maths and motor deficits are also common. These cognitive problems have been shown to be stable into adulthood and do not get worse unlike some of the other physical symptoms of NF-1.[42]
Diagnosis[edit | edit source]
NF1 is often diagnosed early on in childhood.[9]
Disorders that have overlapping features with neurofibromatosis type 1 should be considered in the differential diagnosis, which can include Legius syndrome, skin hyperpigmentation, mismatch repair and overgrowth syndromes and from tumours that are misidentified as neurofibromas (lipomas). Mutations in SPRED1 cause Legius syndrome, which is characterized by CALMs, skinfold freckling, learning difficulties and macrocephaly, but not neurofibromas, Lisch nodules or central nervous system tumours.[24][43] Genetic testing can help to differentiate between a diagnosis of neurofibromatosis type 1 and Legius syndrome. Importantly, neurofibromatosis type 1 is clinically and genetically distinct from other rare tumour predisposition conditions, such as neurofibromatosis type 2 (which is caused by mutations in NF2)[24][44] and from schwannomatosis (which is associated with mutations in SMARCB1 or LZTR1)101,102.[24][45]
The National Institute of Health (NIH) has created specific criteria for the diagnosis of NF-1. Two of these seven "Cardinal Clinical Features" are required for positive diagnosis.[46]
- 6 or more café-au-lait macules over 5 mm in greatest diameter in pre-pubertal individuals and over 15 mm in greatest diameter in post-pubertal individuals
- 2 or more neurofibromas of any type or 1 plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Optic glioma
- 2 or more Lisch nodules (iris harmartomas)
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of the long bone cortex with or without pseudarthrosis
- A first degree relative (parent, sibling, or offspring) with NF1 by the above criteria
DIfferentiating Neurofibromatosis type 1 from other diseases[edit | edit source]
| Disease | Gene | Chromosome | Differentiating Features | Components of MEN | Diagnosis | ||
|---|---|---|---|---|---|---|---|
| Parathyroid | Pitutary | Pancreas | |||||
| von Hippel-Lindau syndrome | Von Hippel–Lindau tumor suppressor | 3p25.3 |
|
- | - | + |
|
| Carney complex | PRKAR1A | 17q23-q24 |
|
- | - | - |
|
| Neurofibromatosis type 1 | RAS | 17 | - | - | - | Prenatal
Postnatal Cardinal Clinical Features" are required for positive diagnosis.
| |
| Li-Fraumeni syndrome | TP53 | 17 | Early onset of diverse amount of cancers such as | - | - | - |
Criteria
|
| Gardner's syndrome | APC | 5q21 |
|
- | - | - |
|
| Multiple endocrine neoplasia type 2 | RET | - |
|
+ | - | - |
Criteria Two or more specific endocrine tumors
|
| Cowden syndrome | PTEN | - | Hamartomas | - | - | - |
|
| Acromegaly/gigantism | - | - |
|
- | + | - |
|
| Pituitary adenoma | - | - |
|
- | + | - |
|
| Hyperparathyroidism | - | - | - | + | - | - |
|
| Pheochromocytoma/paraganglioma |
VHL RET NF1 SDHB SDHD |
- | Characterized by | - | - | - |
|
| Adrenocortical carcinoma |
|
17p, 13q |
|
- | - | - |
|
| Adapted from Toledo SP, Lourenço DM, Toledo RA. A differential diagnosis of inherited endocrine tumors and their tumor counterparts, journal=Clinics (Sao Paulo), volume= 68, issue= 7, 07/24/2013[47] | |||||||
Prognosis[edit | edit source]
There is wide variability in how different individuals with the NF-1 gene manifest the disorder. Some individuals may have no symptoms, while others may have rapidly progressive disorder.
The primary problem of NF-1 is the disfigurement due to the cutaneous neurofibromas, pigmented lesions, and occasional limb abnormalities.
Several more severe complications of NF-1 are listed in the following section.
Complications[edit | edit source]
- Chronic pain, numbness, Pritchetts face, and/or paralysis due to the peripheral nerve sheath tumors
- Blindness due to optic nerve gliomas
- Brain tumors
- Neurologic impairment due to severe spinal scoliosis and/or kyphosis
- Amputation due to a tibial pseudarthrosis
- Malignant degeneration of a plexiform neurofibroma into malignant periphreal nerve sheath tumor (MPNST), occurring in 10-12%
- Depression, It is very common of NF sufferers to suffer severe depression because of the disfigurement it can cause to the body and face.
- Social Anxiety is also common among NF sufferers because of the reaction of others to the condition.
Therapy[edit | edit source]
Therapy for a patient with neurofibromatosis type I is aimed at palliating symptoms and improving quality of life. Treatment modalities offered may include:
- Radiation therapy
- Chemotherapy
- Surgical resection or decompression of an enlarging lesion
- The third face transplant was performed on a patient with this condition.[48]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 Cimino PJ, Gutmann DH (2018). "Neurofibromatosis type 1". Handb Clin Neurol. 148: 799–811. doi:10.1016/B978-0-444-64076-5.00051-X. PMID 29478615.
- ↑
- ↑ Madigan P, Shaw RV (1988). "Neurofibromatosis in 13th century Austria?". Neurofibromatosis. 1 (5–6): 339–41. PMID 3152487.
- ↑ 4.0 4.1 4.2 4.3 4.4 Antonio, Joao Roberto; Goloni-Bertollo, Eny Maria; Tridico, Livia Arroyo (2013). "Neurofibromatosis: chronological history and current issues". Anais Brasileiros de Dermatologia. 88 (3): 329–343. doi:10.1590/abd1806-4841.20132125. ISSN 0365-0596.
- ↑ 5.0 5.1 5.2 5.3 Wander JV, Das Gupta TK (February 1977). "Neurofibromatosis". Curr Probl Surg. 14 (2): 1–81. doi:10.1016/s0011-3840(77)80002-6. PMID 405178.
- ↑ 6.0 6.1 Brosius S (October 2010). "A history of von Recklinghausen's NF1". J Hist Neurosci. 19 (4): 333–48. doi:10.1080/09647041003642885. PMID 20938857.
- ↑ Wander JV, Das Gupta TK (February 1977). "Neurofibromatosis". Curr Probl Surg. 14 (2): 1–81. doi:10.1016/s0011-3840(77)80002-6. PMID 405178.
- ↑
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 Rad E, Tee AR (April 2016). "Neurofibromatosis type 1: Fundamental insights into cell signalling and cancer". Semin. Cell Dev. Biol. 52: 39–46. doi:10.1016/j.semcdb.2016.02.007. PMID 26860753.
- ↑ Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK, Culver M, Carey JC, Copeland NG, Jenkins NA (July 1990). "Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus". Cell. 62 (1): 187–92. doi:10.1016/0092-8674(90)90252-a. PMID 1694727.
- ↑ Daston MM, Scrable H, Nordlund M, Sturbaum AK, Nissen LM, Ratner N (March 1992). "The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes". Neuron. 8 (3): 415–28. doi:10.1016/0896-6273(92)90270-n. PMID 1550670.
- ↑ Nielsen GP, Stemmer-Rachamimov AO, Ino Y, Moller MB, Rosenberg AE, Louis DN (December 1999). "Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation". Am. J. Pathol. 155 (6): 1879–84. doi:10.1016/S0002-9440(10)65507-1. PMC 1866954. PMID 10595918.
- ↑ 13.0 13.1 "oraprdnt.uqtr.uquebec.ca" (PDF).
- ↑ 14.0 14.1 "hekint.org".
- ↑ "Joseph Merrick - - Biography".
- ↑ "Mony Yassir | Degrassi Wiki | Fandom".
- ↑ "Gillian Anderson - Wikipedia, la enciclopedia libre".
- ↑ 18.0 18.1 "4 Famous People With Neurofibromatosis (Alexander Owens)".
- ↑ Seminog OO, Goldacre MJ (January 2013). "Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study". Br. J. Cancer. 108 (1): 193–8. doi:10.1038/bjc.2012.535. PMC 3553528. PMID 23257896.
- ↑ Upadhyaya, Meena; Cooper, David N. (2012). doi:10.1007/978-3-642-32864-0. Missing or empty
|title=(help) - ↑ Peltonen S, Kallionpää RA, Peltonen J (July 2017). "Neurofibromatosis type 1 (NF1) gene: Beyond café au lait spots and dermal neurofibromas". Exp. Dermatol. 26 (7): 645–648. doi:10.1111/exd.13212. PMID 27622733.
- ↑ 22.0 22.1 Trovó-Marqui AB, Tajara EH (July 2006). "Neurofibromin: a general outlook". Clin. Genet. 70 (1): 1–13. doi:10.1111/j.1399-0004.2006.00639.x. PMID 16813595.
- ↑ 23.0 23.1 Boyd KP, Korf BR, Theos A (July 2009). "Neurofibromatosis type 1". J. Am. Acad. Dermatol. 61 (1): 1–14, quiz 15–6. doi:10.1016/j.jaad.2008.12.051. PMC 2716546. PMID 19539839.
- ↑ 24.00 24.01 24.02 24.03 24.04 24.05 24.06 24.07 24.08 24.09 24.10 24.11 24.12 24.13 24.14 24.15 24.16 24.17 Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ (February 2017). "Neurofibromatosis type 1". Nat Rev Dis Primers. 3: 17004. doi:10.1038/nrdp.2017.4. PMID 28230061.
- ↑ Harrisingh, Marie C.; Lloyd, Alison C. (2014). "Ras/Raf/ERK signalling and NF1: Implications for Neurofibroma Formation". Cell Cycle. 3 (10): 1255–1258. doi:10.4161/cc.3.10.1182. ISSN 1538-4101.
- ↑ Wang, Yuan; Kim, Edward; Wang, Xiaojing; Novitch, Bennett G.; Yoshikawa, Kazuaki; Chang, Long-Sheng; Zhu, Yuan (2012). "ERK Inhibition Rescues Defects in Fate Specification of Nf1-Deficient Neural Progenitors and Brain Abnormalities". Cell. 150 (4): 816–830. doi:10.1016/j.cell.2012.06.034. ISSN 0092-8674.
- ↑ Dodd KM, Yang J, Shen MH, Sampson JR, Tee AR (April 2015). "mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3". Oncogene. 34 (17): 2239–50. doi:10.1038/onc.2014.164. PMC 4172452. PMID 24931163.
- ↑ Messiaen LM, Callens T, Mortier G, Beysen D, Vandenbroucke I, Van Roy N, Speleman F, Paepe AD (2000). "Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects". Hum. Mutat. 15 (6): 541–55. doi:10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. PMID 10862084.
- ↑ DeClue JE, Cohen BD, Lowy DR (November 1991). "Identification and characterization of the neurofibromatosis type 1 protein product". Proc. Natl. Acad. Sci. U.S.A. 88 (22): 9914–8. doi:10.1073/pnas.88.22.9914. PMC 52837. PMID 1946460.
- ↑ Friedrich RE, Schmelzle R, Hartmann M, Fünsterer C, Mautner VF (January 2005). "Resection of small plexiform neurofibromas in neurofibromatosis type 1 children". World J Surg Oncol. 3 (1): 6. doi:10.1186/1477-7819-3-6. PMC 549083. PMID 15683544.
- ↑ "Neurofibromatosis type II - Wikipedia".
- ↑ 32.0 32.1 De Schepper S, Maertens O, Callens T, Naeyaert JM, Lambert J, Messiaen L (April 2008). "Somatic mutation analysis in NF1 café au lait spots reveals two NF1 hits in the melanocytes". J. Invest. Dermatol. 128 (4): 1050–3. doi:10.1038/sj.jid.5701095. PMID 17914445.
- ↑ 33.0 33.1 33.2 33.3 33.4 33.5 Cimino PJ, Gutmann DH (2018). "Neurofibromatosis type 1". Handb Clin Neurol. 148: 799–811. doi:10.1016/B978-0-444-64076-5.00051-X. PMID 29478615.
- ↑ 34.0 34.1 "Clinical and genetic patterns of neurofibromatosis 1 and 2. | British Journal of Ophthalmology".
- ↑ . doi:10.1016/j.jpeds.2006.10.048Get rights and content Objective Check
|doi=value (help). Missing or empty|title=(help) - ↑ Rodriguez FJ, Folpe AL, Giannini C, Perry A (March 2012). "Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems". Acta Neuropathol. 123 (3): 295–319. doi:10.1007/s00401-012-0954-z. PMC 3629555. PMID 22327363.
- ↑ Lewis, Richard Alan; Gerson, L. Paul; Axelson, Kenneth A.; Riccardi, Vincent M.; Whitford, Randolph P. (1984). "von Recklinghausen Neurofibromatosis". Ophthalmology. 91 (8): 929–935. doi:10.1016/S0161-6420(84)34217-8. ISSN 0161-6420.
- ↑ Rosser, T. L.; Vezina, G.; Packer, R. J. (2005). "Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1". Neurology. 64 (3): 553–555. doi:10.1212/01.WNL.0000150544.00016.69. ISSN 0028-3878.
- ↑ Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH (December 2003). "Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity". Cancer Res. 63 (24): 8573–7. PMID 14695164.
- ↑ "Neurofibromatosis - Orthopaedics and Trauma".
- ↑ Hyman, SL. et al.(2005). The Nature and Frequency of Cognitive Deficits in Children with Neurofibromatosis Type 1. Neurology, 65, 1037-1044.
- ↑ Hyman, S.L. et al. (2003). Natural History of Neuropsychological Ability and T2-Hyperintensities in Patients with Neurofibromatosis Type 1. Neurology, 60(7), 1139-1145.
- ↑ Messiaen L, Yao S, Brems H, Callens T, Sathienkijkanchai A, Denayer E, Spencer E, Arn P, Babovic-Vuksanovic D, Bay C, Bobele G, Cohen BH, Escobar L, Eunpu D, Grebe T, Greenstein R, Hachen R, Irons M, Kronn D, Lemire E, Leppig K, Lim C, McDonald M, Narayanan V, Pearn A, Pedersen R, Powell B, Shapiro LR, Skidmore D, Tegay D, Thiese H, Zackai EH, Vijzelaar R, Taniguchi K, Ayada T, Okamoto F, Yoshimura A, Parret A, Korf B, Legius E (November 2009). "Clinical and mutational spectrum of neurofibromatosis type 1-like syndrome". JAMA. 302 (19): 2111–8. doi:10.1001/jama.2009.1663. PMID 19920235.
- ↑ Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K (March 1993). "A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor". Cell. 72 (5): 791–800. doi:10.1016/0092-8674(93)90406-g. PMID 8453669.
- ↑ Hulsebos TJ, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P (April 2007). "Germline mutation of INI1/SMARCB1 in familial schwannomatosis". Am. J. Hum. Genet. 80 (4): 805–10. doi:10.1086/513207. PMC 1852715. PMID 17357086.
- ↑ Huson SM, Hughes RAC. The Neurofibromatoses. London, UK: Chapman and Hall; 1994;1.3.2:9
- ↑ Toledo SP, Lourenço DM, Toledo RA (2013). "A differential diagnosis of inherited endocrine tumors and their tumor counterparts". Clinics (Sao Paulo). 68 (7): 1039–56. doi:10.6061/clinics/2013(07)24. PMC 3715026. PMID 23917672.
- ↑ http://today.reuters.com/news/articlenews.aspx?type=healthNews&storyID=2007-01-23T185518Z_01_L23896370_RTRUKOC_0_US-FRANCE-TRANSPLANT.xml
- Cotran R, Kumar V, Robbins S (eds). Robbins Pathologic Basis of Disease, 5th ed. WB Saunders, 1994.
Template:Phakomatoses and other congenital malformations not elsewhere classified
Template:Link FA
de:Neurofibromatose Typ 1
nl:Ziekte van Von Recklinghausen
sr:Неурофиброматоза тип 1
fi:Neurofibromatoosi 1
 KSF
KSF