Nicotine
 From Wikidoc - Reading time: 23 min
From Wikidoc - Reading time: 23 min
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nicorette, Nicotrol |
| AHFS/Drugs.com | Monograph |
| Pregnancy category | |
| Dependence liability | Physical: moderate Psychological: high[1] |
| Addiction liability | Low-moderate |
| Routes of administration | Inhalation; insufflation; oral – buccal, sublingual, and ingestion; transdermal; rectal, |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20 to 45% (oral), 53% (intranasal), 68% (transdermal) |
| Protein binding | <5% |
| Metabolism | Hepatic |
| Elimination half-life | 1-2 hours; 20 hours active metabolite (cotinine) |
| Excretion | Urine (10-20% (gum), pH-dependent; 30% (inhaled); 10-30% (intranasal)) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C10H14N2 |
| Molar mass | 162.23 g/mol |
| 3D model (JSmol) | |
| Density | 1.01 g/cm3 |
| Melting point | −79 °C (−110.2 °F) |
| Boiling point | 247 °C (476.6 °F) |
| |
| |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview[edit | edit source]
Nicotine is a potent parasympathomimetic alkaloid found in the nightshade family of plants (Solanaceae) and a stimulant drug. Nicotine is a nicotinic acetylcholine receptor (nAChR) agonist,[2][3] except at nAChRα9 and nAChRα10 where it acts as an antagonist.[2] It is made in the roots of and accumulates in the leaves of the nightshade family of plants. It constitutes approximately 0.6–3.0% of the dry weight of tobacco[4] and is present in the range of 2–7 µg/kg of various edible plants.[5] It functions as an antiherbivore chemical; consequently, nicotine was widely used as an insecticide in the past[6][7] and nicotine analogs such as imidacloprid are currently widely used.
In lesser doses (an average cigarette yields about 2 mg of absorbed nicotine), the substance acts as a stimulant in mammals, while high amounts (50–100 mg) can be harmful.[8][9][10] This stimulant effect is a major contributing factor to the addictive properties of tobacco smoking.
Uses[edit | edit source]
Medical[edit | edit source]
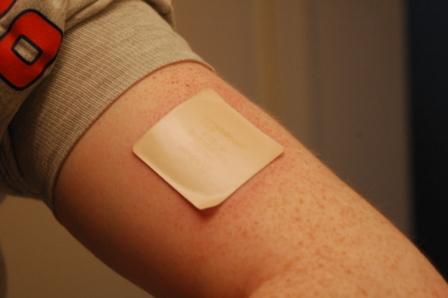
The primary therapeutic use of nicotine is in treating nicotine dependence in order to eliminate smoking with the damage it does to health. Controlled levels of nicotine are given to patients through gums, dermal patches, lozenges, electronic/substitute cigarettes or nasal sprays in an effort to wean them off their dependence.
Studies have found that these therapies increase the chance of success of quitting by 50 to 70%,[11] though reductions in the population as a whole has not been demonstrated.[12]
Enhancing performance[edit | edit source]
Nicotine is frequently used for its performance enhancing effects on cognition, alertness and focus.[13][14] It is second only to caffeine as the most widely used nootropic in the world.[citation needed]
Recreational[edit | edit source]
Nicotine is commonly consumed as a recreational drug for its stimulant effects.[15]
Psychoactive effects[edit | edit source]
Nicotine's mood-altering effects are different by report: in particular it is both a stimulant and a relaxant.[16] First causing a release of glucose from the liver and epinephrine (adrenaline) from the adrenal medulla, it causes stimulation. Users report feelings of relaxation, sharpness, calmness, and alertness.[17] Like any stimulant, it may very rarely cause the often uncomfortable neuropsychiatric effect of akathisia. By reducing the appetite and raising the metabolism, some smokers may lose weight as a consequence.[18][19]
When a cigarette is smoked, nicotine-rich blood passes from the lungs to the brain within seven seconds and immediately stimulates the release of many chemical messengers such as acetylcholine, norepinephrine, epinephrine, arginine vasopressin, serotonin, dopamine, and beta-endorphin.[20][21] This release of neurotransmitters and hormones is responsible for most of nicotine's psychoactive effects. Nicotine appears to enhance concentration[22] and memory due to the increase of acetylcholine. It also appears to enhance alertness due to the increases of acetylcholine and norepinephrine. Arousal is increased by the increase of norepinephrine. Pain is reduced by the increases of acetylcholine and beta-endorphin. Anxiety is reduced by the increase of beta-endorphin. Nicotine also extends the duration of positive effects of dopamine[23] and increases sensitivity in brain reward systems.[24] Most cigarettes (in the smoke inhaled) contain 1 to 3 milligrams of nicotine.[25]
Studies suggest that when smokers wish to achieve a stimulating effect, they take short quick puffs, which produce a low level of blood nicotine.[26] This stimulates nerve transmission. When they wish to relax, they take deep puffs, which produce a higher level of blood nicotine, which depresses the passage of nerve impulses, producing a mild sedative effect. At low doses, nicotine potently enhances the actions of norepinephrine and dopamine in the brain, causing a drug effect typical of those of psychostimulants. At higher doses, nicotine enhances the effect of serotonin and opiate activity, producing a calming, pain-killing effect. Nicotine is unique in comparison to most drugs, as its profile changes from stimulant to sedative/pain killer in increasing dosages and use, a phenomenon described by Paul Nesbitt in his doctoral dissertation[27] and subsequently referred to as "Nesbitt's Paradox".[28]
Adverse effects[edit | edit source]
Vascular system[edit | edit source]

Nicotine increases blood pressure and heart rate.[29] Nicotine can also induce potentially atherogenic genes in human coronary artery endothelial cells.[30] Microvascular injury can result through its action on nicotinic acetylcholine receptors (nAChRs).[31]
Carcinogen[edit | edit source]
Historically, nicotine has not been regarded as a carcinogen.[32] The IARC has not evaluated nicotine in its standalone form or assigned it to an official carcinogen group. While no epidemiological evidence supports that nicotine alone acts as a carcinogen in the formation of human cancer, research over the last decade has identified nicotine's carcinogenic potential in animal models and cell culture.[33][34][35] Indirectly, nicotine increases cholinergic signalling (and adrenergic signalling in the case of colon cancer[36]), thereby impeding apoptosis (programmed cell death), promoting tumor growth, and activating growth factors and cellular mitogenic factors such as 5-LOX, and EGF. Nicotine also promotes cancer growth by stimulating angiogenesis and neovascularization.[37][38] In one study, nicotine administered to mice with tumors caused increases in tumor size (twofold increase), metastasis (nine-fold increase), and tumor recurrence (threefold increase).[39] N-Nitrosonornicotine (NNN), classified by the IARC as a Group 1 carcinogen, has been shown to form in vitro in amounts less than 0.01% the active substance, when human saliva is incurbated with nornicotine.[40]
Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis.[41]
Fetal development[edit | edit source]
In pregnancy, a 2013 review noted that "nicotine is only 1 of more than 4000 compounds to which the fetus is exposed through maternal smoking. Of these, ∼30 compounds have been associated with adverse health outcomes. Although the exact mechanisms by which nicotine produces adverse fetal effects are unknown, it is likely that hypoxia, undernourishment of the fetus, and direct vasoconstrictor effects on the placental and umbilical vessels all play a role. Nicotine also has been shown to have significant deleterious effects on brain development, including alterations in brain metabolism and neurotransmitter systems and abnormal brain development." It also notes that "abnormalities of newborn neurobehavior, including impaired orientation and autonomic regulation and abnormalities of muscle tone, have been identified in a number of prenatal nicotine exposure studies" and that there is weak data associating fetal nicotine exposure with newborn facial clefts, and that there is no good evidence for newborns suffering nicotine withdrawal from fetal exposure to nicotine.[42]
Effective April 1, 1990, the Office of Environmental Health Hazard Assessment (OEHHA) of the California Environmental Protection Agency added nicotine to the list of chemicals known to cause developmental toxicity.[43]
Dependence and withdrawal[edit | edit source]
Difficulty concentrating and deficits in task performance are symptoms of nicotine withdrawal. These symptoms begin as soon as 30 minutes after tobacco cessation begins, and can last for several weeks.[44]
Nicotine appears to have significant performance enhancing effects, particularly in fine motor skills, attention, and memory. These beneficial cognitive effects may play a role in the initiation and maintenance of tobacco dependence.[44]
Studies suggest a correlation between smoking and schizophrenia, with estimates near 75% for the proportion of schizophrenic patients who smoke. Although the nature of this association remains unclear, it has been argued that the increased level of smoking in schizophrenia may be due to a desire to self-medicate with nicotine.[45][46] Other research found that mildly dependent users got some benefit from nicotine, but not those who were highly dependent.[47]
Overdose[edit | edit source]
The Template:LD50 of nicotine is 50 mg/kg for rats and 3 mg/kg for mice. 30–60 mg (0.5–1.0 mg/kg) can be a lethal dosage for adult humans.[8][48] However the widely used human LD50 estimate of 0.5–1.0 mg/kg was questioned in a 2013 review, in light of several documented cases of humans surviving much higher doses; the 2013 review suggests that the lower limit causing fatal outcomes is 500–1000 mg of ingested nicotine, corresponding to 6.5–13 mg/kg orally.[10] Nevertheless nicotine has a relatively high toxicity in comparison to many other alkaloids such as caffeine, which has an LD50of 127 mg/kg when administered to mice.[49]
It is unlikely that a person would overdose on nicotine through smoking alone, the US Food and Drug Administration (FDA) states in 2013 "There are no significant safety concerns associated with using more than one OTC NRT at the same time, or using an OTC NRT at the same time as another nicotine-containing product—including a cigarette."[50] Spilling a high concentration of nicotine onto the skin can cause intoxication or even death, since nicotine readily passes into the bloodstream following dermal contact.[51]
Addiction[edit | edit source]
Nicotine is addictive.[52][53] Nicotine activates the mesolimbic pathway and induces long-term ΔFosB expression in the nucleus accumbens when inhaled or injected, but not necessarily when ingested.[52][53][54] Consequently, repeated daily exposure (possibly excluding oral route) to nicotine can result in accumbal ΔFosB overexpression, in turn causing nicotine addiction.[52][53]
Pharmacology[edit | edit source]
Pharmacodynamics[edit | edit source]
Central nervous system[edit | edit source]

By binding to nicotinic acetylcholine receptors, nicotine increases the levels of several neurotransmitters – acting as a sort of "volume control". It is thought that increased levels of dopamine in the reward circuits of the brain are a major contributor to the apparent euphoria and relaxation, and addiction caused by nicotine consumption. Nicotine-induced dopamine release occurs via the cholinergic–dopaminergic reward link, which is mediated by the neuropeptide ghrelin in the ventral tegmentum.[55] Nicotine has a higher affinity for acetylcholine receptors in the brain than those in skeletal muscle, though at toxic doses it can induce contractions and respiratory paralysis.[56] Nicotine's selectivity is thought to be due to a particular amino acid difference on these receptor subtypes.[57]
Tobacco smoke contains anabasine, anatabine, and nornicotine. It also contains the monoamine oxidase inhibitors harman and norharman.[58] These beta-carboline compounds significantly decrease MAO activity in smokers.[58][59] MAO enzymes break down monoaminergic neurotransmitters such as dopamine, norepinephrine, and serotonin. It is thought that the powerful interaction between the MAOIs and the nicotine is responsible for most of the addictive properties of tobacco smoking.[60] The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats.[61]
Chronic nicotine exposure via tobacco smoking up-regulates alpha4beta2* nAChR in cerebellum and brainstem regions[62][63] but not habenulopeduncular structures.[64] Alpha4beta2 and alpha6beta2 receptors, present in the ventral tegmental area, play a crucial role in mediating the reinforcement effects of nicotine.[65]
Research published in 2011 found that nicotine inhibits class I and II histone deacetylases, chromatin-modifying enzymes involved in epigenetics. This inhibition has been shown to increase susceptibility to cocaine addiction in rodents.[66][67]
Sympathetic nervous system[edit | edit source]
Nicotine also activates the sympathetic nervous system,[68] acting via splanchnic nerves to the adrenal medulla, stimulating the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing the release of epinephrine (and noradrenaline) into the bloodstream. Nicotine also has an affinity for melanin-containing tissues due to its precursor function in melanin synthesis or due to the irreversible binding of melanin and nicotine. This has been suggested to underlie the increased nicotine dependence and lower smoking cessation rates in darker pigmented individuals. However, further research is warranted before a definite conclusive link can be inferred.[69]
Adrenal medulla[edit | edit source]

By binding to ganglion type nicotinic receptors in the adrenal medulla, nicotine increases flow of adrenaline (epinephrine), a stimulating hormone and neurotransmitter. By binding to the receptors, it causes cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and thus the release of epinephrine (and norepinephrine) into the bloodstream. The release of epinephrine (adrenaline) causes an increase in heart rate, blood pressure and respiration, as well as higher blood glucose levels.[70]
Nicotine has a half-life of 1 to 2 hours. Cotinine is an active metabolite of nicotine that remains in the blood for 18 to 20 hours, making it easier to analyze due to its longer half-life.[71]
Pharmacokinetics[edit | edit source]
As nicotine enters the body, it is distributed quickly through the bloodstream and crosses the blood–brain barrier reaching the brain within 10–20 seconds after inhalation.[72] The elimination half-life of nicotine in the body is around two hours.[73]
The amount of nicotine absorbed by the body from smoking can depend on many factors, including the types of tobacco, whether the smoke is inhaled, and whether a filter is used. However, it has been found that the nicotine yield of individual products has only a small effect (4.4%) on the blood concentration of nicotine,[74] suggesting "the assumed health advantage of switching to lower-tar and lower-nicotine cigarettes may be largely offset by the tendency of smokers to compensate by increasing inhalation".
Nicotine acts on nicotinic acetylcholine receptors, specifically the α3β4 ganglion type nicotinic receptor, present in the autonomic ganglia and adrenal medulla, and a central nervous system (CNS) α4β2 nicotinic receptor. In small concentrations, nicotine increases the activity of these cholinergic receptors and indirectly on a variety of other neurotransmitters such as dopamine.
Nicotine is metabolized in the liver by cytochrome P450 enzymes (mostly CYP2A6, and also by CYP2B6). A major metabolite is cotinine. Other primary metabolites include nicotine N'-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide.[75] Under some conditions, other substances may be formed such as myosmine.[76]
Glucuronidation and oxidative metabolism of nicotine to cotinine are both inhibited by menthol, an additive to mentholated cigarettes, thus increasing the half-life of nicotine in vivo.[77]
Physical and chemical properties[edit | edit source]
Template:NFPA 704 Nicotine is a hygroscopic, colorless oily liquid that is readily soluble in alcohol, ether or light petroleum. It is miscible with water in its base form between 60 °C and 210 °C. As a nitrogenous base, nicotine forms salts with acids that are usually solid and water soluble. Its flash point is 95 °C and its auto-ignition temperature is 244 °C.[78]
Nicotine is optically active, having two enantiomeric forms. The naturally occurring form of nicotine is levorotatory with a specific rotation of [α]D = –166.4° ((−)-nicotine). The dextrorotatory form, (+)-nicotine is physiologically less active than (–)-nicotine. (−)-nicotine is more toxic than (+)-nicotine.[79] The salts of (+)-nicotine are usually dextrorotatory. The hydrochloride and sulphate salts become optically inactive if heated in a closed vessel above 180 °C.[80]
On exposure to ultraviolet light or various oxidizing agents, nicotine is converted to nicotine oxide, nicotinic acid (vitamin B3), and methylamine.[80]
Occurrence and biosynthesis[edit | edit source]

Nicotine is a natural product of tobacco, occurring in the leaves in a range of 0.5 to 7.5% depending on variety.[81] Nicotine also naturally occurs in smaller amounts in plants from the family Solanaceae (such as potatoes, tomatoes, and eggplant).[82]
The biosynthetic pathway of nicotine involves a coupling reaction between the two cyclic structures that compose nicotine. Metabolic studies show that the pyridine ring of nicotine is derived from niacin (nicotinic acid) while the pyrrolidone is derived from N-methyl-Δ1-pyrrollidium cation.[83][84] Biosynthesis of the two component structures proceeds via two independent syntheses, the NAD pathway for niacin and the tropane pathway for N-methyl-Δ1-pyrrollidium cation.
The NAD pathway in the genus nicotiana begins with the oxidation of aspartic acid into α-imino succinate by aspartate oxidase (AO). This is followed by a condensation with glyceraldehyde-3-phosphate and a cyclization catalyzed by quinolinate synthase (QS) to give quinolinic acid. Quinolinic acid then reacts with phosphoriboxyl pyrophosphate catalyzed by quinolinic acid phosphoribosyl transferase (QPT) to form niacin mononucleotide (NaMN). The reaction now proceeds via the NAD salvage cycle to produce niacin via the conversion of nicotinamide by the enzyme nicotinamidase.[citation needed]
The N-methyl-Δ1-pyrrollidium cation used in the synthesis of nicotine is an intermediate in the synthesis of tropane-derived alkaloids. Biosynthesis begins with decarboxylation of ornithine by ornithine decarboxylase (ODC) to produce putrescine. Putrescine is then converted into N-methyl putrescine via methylation by SAM catalyzed by putrescine N-methyltransferase (PMT). N-methylputrescine then undergoes deamination into 4-methylaminobutanal by the N-methylputrescine oxidase (MPO) enzyme, 4-methylaminobutanal then spontaneously cyclize into N-methyl-Δ1-pyrrollidium cation.[citation needed]
The final step in the synthesis of nicotine is the coupling between N-methyl-Δ1-pyrrollidium cation and niacin. Although studies conclude some form of coupling between the two component structures, the definite process and mechanism remains undetermined. The current agreed theory involves the conversion of niacin into 2,5-dihydropyridine through 3,6-dihydronicotinic acid. The 2,5-dihydropyridine intermediate would then react with N-methyl-Δ1-pyrrollidium cation to form enantiomerically pure (–)-nicotine.[85]
Measurement in body fluids[edit | edit source]
Nicotine can be quantified in blood, plasma, or urine to confirm a diagnosis of poisoning or to facilitate a medicolegal death investigation. Urinary or salivary cotinine concentrations are frequently measured for the purposes of pre-employment and health insurance medical screening programs. Careful interpretation of results is important, since passive exposure to cigarette smoke can result in significant accumulation of nicotine, followed by the appearance of its metabolites in various body fluids.[86][87] Nicotine use is not regulated in competitive sports programs.[88]
History[edit | edit source]
Nicotine is named after the tobacco plant Nicotiana tabacum, which in turn is named after the French ambassador in Portugal, Jean Nicot de Villemain, who sent tobacco and seeds to Paris in 1560, presented to the French King,[89] and who promoted their medicinal use. The tobacco and its seeds were brought to Ambassador Nicot from Brazil by Luis de Gois, a Portuguese colonist in São Paulo.[citation needed]Smoking was believed to protect against illness, particularly the plague.[89]
Tobacco was introduced to Europe in 1559, and by the late 17th century, it was used not only for smoking but also as an insecticide. After World War II, over 2,500 tons of nicotine insecticide were used worldwide, but by the 1980s the use of nicotine insecticide had declined below 200 tons. This was due to the availability of other insecticides that are cheaper and less harmful to mammals.[7]
Currently, nicotine, even in the form of tobacco dust, is prohibited as a pesticide for organic farming in the United States.[90][91]
In 2008, the EPA received a request, from the registrant, to cancel the registration of the last nicotine pesticide registered in the United States.[92] This request was granted, and since 1 January 2014, this pesticide has not been available for sale.[93]
Chemical identification[edit | edit source]
Nicotine was first isolated from the tobacco plant in 1828 by physician Wilhelm Heinrich Posselt and chemist Karl Ludwig Reimann of Germany, who considered it a poison.[94][95] Its chemical empirical formula was described by Melsens in 1843,[96] its structure was discovered by Adolf Pinner and Richard Wolffenstein in 1893,[97][98][99][clarification needed] and it was first synthesized by Amé Pictet and A. Rotschy in 1904.[100]
Society and culture[edit | edit source]
The nicotine content of popular American-brand cigarettes has slowly increased over the years, and one study found that there was an average increase of 1.78% per year between the years of 1998 and 2005.[101]
Research[edit | edit source]
While acute/initial nicotine intake causes activation of nicotine receptors, chronic low doses of nicotine use leads to desensitisation of nicotine receptors (due to the development of tolerance) and results in an antidepressant effect, with research showing low dose nicotine patches being an effective treatment of major depressive disorder in non-smokers.,[102] However the original research concluded that: "Nicotine patches produced short-term improvement of depression with minor side effects. Because of nicotine's high risk to health, nicotine patches are not recommended for clinical use in depression."[103]
Though tobacco smoking is associated with an increased risk of Alzheimer's disease,[104] there is evidence that nicotine itself has the potential to prevent and treat Alzheimer's disease.[105]
Research into nicotine's most predominant metabolite, cotinine, suggests that some, if not most, of nicotine's psychoactive effects may actually be mediated by complex interactions with cotinine, or perhaps even by cotinine alone rather than strictly by nicotine as conventionally thought.[106][107]
Little research is available in humans but animal research suggests there is potential benefit from nicotine in Parkinson's disease.[108]
See also[edit | edit source]
References[edit | edit source]
- ↑ Cosci, F; Pistelli, F; Lazzarini, N; Carrozzi, L (2011). "Nicotine dependence and psychological distress: outcomes and clinical implications in smoking cessation". Psychology research and behavior management. 4: 119–28. doi:10.2147/prbm.s14243. PMID 22114542.
- ↑ 2.0 2.1 "Nicotinic acetylcholine receptors: Introduction". IUPHAR Database. International Union of Basic and Clinical Pharmacology. Retrieved 1 September 2014.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 9: Autonomic Nervous System". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 234. ISBN 9780071481274.
Nicotine ... is a natural alkaloid of the tobacco plant. Lobeline is a natural alkaloid of Indian tobacco. Both drugs are agonists are nicotinic cholinergic receptors ...
- ↑ "Smoking and Tobacco Control Monograph No. 9" (PDF). Retrieved 2012-12-19.
- ↑ "Determination of the Nicotine Content of Various Edible Nightshades (Solanaceae) and Their Products and Estimation of the Associated Dietary Nicotine Intake". Retrieved 2008-10-05.
- ↑ Rodgman, Alan; Perfetti, Thomas A. (2009). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press. ISBN 1-4200-7883-6. LCCN 2008018913.[page needed]
- ↑ 7.0 7.1 Ujváry, István (1999). "Nicotine and Other Insecticidal Alkaloids". In Yamamoto, Izuru; Casida, John. Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo: Springer-Verlag. pp. 29–69.
- ↑ 8.0 8.1 "Nicotine (PIM)". Inchem.org. Retrieved 2012-12-19.
- ↑ Genetic Science Learning Center. "How Drugs Can Kill".
- ↑ 10.0 10.1 Mayer B (January 2014). "How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century". Archives of Toxicology. 88 (1): 5–7. doi:10.1007/s00204-013-1127-0. PMC 3880486. PMID 24091634.
- ↑ 11.0 11.1 Stead LF, Perera R, Bullen C, Mant D, Lancaster T (2008). Stead, Lindsay F, ed. "Nicotine replacement therapy for smoking cessation". Cochrane Database Syst Rev (1): CD000146. doi:10.1002/14651858.CD000146.pub3. PMID 18253970.
- ↑ Pierce, John P.; Cummins, Sharon E.; White, Martha M.; Humphrey, Aimee; Messer, Karen (2012). "Quitlines and Nicotine Replacement for Smoking Cessation: Do We Need to Change Policy?". Annual Review of Public Health. 33: 341–56. doi:10.1146/annurev-publhealth-031811-124624. PMID 22224888.
- ↑ Jasinska, Agnes J.; Zorick, Todd; Brody, Arthur L.; Stein, Elliot A. (September 2014). "Dual role of nicotine in addiction and cognition: A review of neuroimaging studies in humans". Neuropharmacology. 84: 111–122. doi:10.1016/j.neuropharm.2013.02.015. Retrieved 28 April 2015.
- ↑ Heishman SJ, Kleykamp BA, Singleton EG (June 2010). "Meta-analysis of the acute effects of nicotine and smoking on human performance". Psychopharmacology (Berl). 210 (4): 453–69. doi:10.1007/s00213-010-1848-1. PMC 3151730. PMID 20414766.
|access-date=requires|url=(help) - ↑ "DrugFacts: Cigarettes and Other Tobacco Products". National Institute on Drug Abuse. December 2014. Retrieved 28 April 2015.
- ↑ "Effective Clinical Tobacco Intervention". Therapeutics Letter (21): 1–4. September–October 1997.
- ↑ Lagrue, Gilbert; Cormier, Anne (June 2001). "Des récepteurs nicotiniques à la dépendance tabagique : Perspectives thérapeutiques". Alcoologie et addictologie (in français). 23 (2): 39S–42S. ISSN 1620-4522. INIST:1081618. Unknown parameter
|trans_title=ignored (help) - ↑ Orsini, Jean-Claude (June 2001). "Dépendance tabagique et contrôle central de la glycémie et de l'appétit". Alcoologie et addictologie (in français). 23 (2 Suppl): 28S–36S. ISSN 1620-4522. INIST:1081638. Unknown parameter
|trans_title=ignored (help) - ↑ Chen, Hui; Vlahos, Ross; Bozinovski, Steve; Jones, Jessica; Anderson, Gary P; Morris, Margaret J (2004). "Effect of Short-Term Cigarette Smoke Exposure on Body Weight, Appetite and Brain Neuropeptide Y in Mice". Neuropsychopharmacology. 30 (4): 713–9. doi:10.1038/sj.npp.1300597. PMID 15508020. Lay summary – The University of Melbourne (1 November 2004).
- ↑ Pomerleau OF, Pomerleau CS (1984). "Neuroregulators and the reinforcement of smoking: Towards a biobehavioral explanation". Neuroscience and Biobehavioral Reviews. 8: 503–513. doi:10.1016/0149-7634(84)90007-1.
- ↑ Pomerleau OF, Rosecrans J (1989). "Neuroregulatory effects of nicotine". Psychoneuroendocrinology. 14: 407–423. doi:10.1016/0306-4530(89)90040-1.
- ↑ Rusted J, Graupner L, O'Connell N, Nicholls C (August 1994). "Does nicotine improve cognitive function?". Psychopharmacology (Berl.). 115 (4): 547–9. doi:10.1007/BF02245580. PMID 7871101.
- ↑ Easton, John (March 28, 2002). "Nicotine extends duration of pleasant effects of dopamine". The University of Chicago Chronicle. 21 (12).
- ↑ Kenny PJ, Markou A (Jun 2006). "Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity". Neuropsychopharmacology. 31 (6): 1203–11. doi:10.1038/sj.npp.1300905. PMID 16192981.
- ↑ "Erowid Nicotine Vault : Dosage". Erowid.org. 2011-10-14. Retrieved 2012-12-19.
- ↑ Golding, J. F.; Mangan, G. L. (1989). "Factors Governing Recruitment to and Maintenance of Smoking". In Einstein, Stanley. Drug and Alcohol Use. pp. 101–17. doi:10.1007/978-1-4899-0888-9_9. ISBN 978-1-4899-0890-2.
- ↑ Nesbitt P (1969). Smoking, physiological arousal, and emotional response. Unpublished doctoral dissertation, Columbia University.
- ↑ Parrott AC (January 1998). "Nesbitt's Paradox resolved? Stress and arousal modulation during cigarette smoking". Addiction. 93 (1): 27–39. doi:10.1046/j.1360-0443.1998.931274.x. PMID 9624709.
- ↑ Sabha M, Tanus-Santos JE, Toledo JC, Cittadino M, Rocha JC, Moreno H (August 2000). "Transdermal nicotine mimics the smoking-induced endothelial dysfunction". Clinical Pharmacology and Therapeutics. 68 (2): 167–74. doi:10.1067/mcp.2000.108851. PMID 10976548.
- ↑ Zhang S, Day I, Ye S (February 2001). "Nicotine induced changes in gene expression by human coronary artery endothelial cells". Atherosclerosis. 154 (2): 277–83. doi:10.1016/S0021-9150(00)00475-5. PMID 11166759.
- ↑ Hawkins BT, Brown RC, Davis TP (February 2002). "Smoking and ischemic stroke: a role for nicotine?". Trends in Pharmacological Sciences. 23 (2): 78–82. doi:10.1016/S0165-6147(02)01893-X. PMID 11830264.
- ↑ Cardinale A, Nastrucci C, Cesario A, Russo P (January 2012). "Nicotine: specific role in angiogenesis, proliferation and apoptosis". Critical Reviews in Toxicology. 42 (1): 68–89. doi:10.3109/10408444.2011.623150. PMID 22050423.
- ↑ Hecht SS (July 1999). "Tobacco smoke carcinogens and lung cancer". J. Natl. Cancer Inst. 91 (14): 1194–210. doi:10.1093/jnci/91.14.1194. PMID 10413421.
- ↑ Wu WK, Cho CH (April 2004). "The pharmacological actions of nicotine on the gastrointestinal tract". J. Pharmacol. Sci. 94 (4): 348–58. doi:10.1254/jphs.94.348. PMID 15107574.
- ↑ Chowdhury P, Udupa KB (December 2006). "Nicotine as a mitogenic stimulus for pancreatic acinar cell proliferation". World J. Gastroenterol. 12 (46): 7428–32. PMID 17167829.
- ↑ Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH (June 2007). "Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation". Toxicol. Sci. 97 (2): 279–87. doi:10.1093/toxsci/kfm060. PMID 17369603.
- ↑ Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M (October 2003). "Nicotine enhances neovascularization and promotes tumor growth". Mol. Cells. 16 (2): 143–6. PMID 14651253.
- ↑ Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH (January 2004). "Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway". J. Pharmacol. Exp. Ther. 308 (1): 66–72. doi:10.1124/jpet.103.058321. PMID 14569062.
- ↑ Davis R, Rizwani W, Banerjee S; et al. (2009). Pao, William, ed. "Nicotine promotes tumor growth and metastasis in mouse models of lung cancer". PLoS ONE. 4 (10): e7524. Bibcode:2009PLoSO...4.7524D. doi:10.1371/journal.pone.0007524. PMC 2759510. PMID 19841737.
- ↑ "Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N'-nitrosonornicotine in humans". PMID 22923602.
- ↑ Heeschen, C (July 2001). "Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis". Nat Med. 7: 833–9. doi:10.1038/89961. PMID 11433349. Retrieved 2 January 2015.
- ↑ Behnke M, Smith VC (March 2013). "Prenatal substance abuse: short- and long-term effects on the exposed fetus". Pediatrics. 131 (3): e1009–24. doi:10.1542/peds.2012-3931. PMID 23439891.
- ↑ http://oehha.ca.gov/prop65/prop65_list/files/P65single121809.pdf[full citation needed]
- ↑ 44.0 44.1 Heishman, SJ, Kleykamp, BA, Singleton, EG (July 2010). "Meta-analysis of the acute effects of nicotine and smoking on human performance". Pharmacology. 210 (4): 453–69. doi:10.1007/s00213-010-1848-1. PMC 3151730. PMID 20414766.
The significant effects of nicotine on motor abilities, attention, and memory likely represent true performance enhancement because they are not confounded by withdrawal relief. The beneficial cognitive effects of nicotine have implications for initiation of smoking and maintenance of tobacco dependence.
- ↑ de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ (Jul 2002). "Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital". Schizophr Res. 56 (1–2): 55–65. doi:10.1016/S0920-9964(01)00192-X. PMID 12084420.
- ↑ de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM (Mar 1995). "Schizophrenia and smoking: an epidemiological survey in a state hospital". Am J Psychiatry. 152 (3): 453–5. PMID 7864277.
- ↑ Aguilar MC, Gurpegui M, Diaz FJ, de Leon J (Mar 2005). "Nicotine dependence and symptoms in schizophrenia: naturalistic study of complex interactions". Br J Psychiatry. 186 (3): 215–21. doi:10.1192/bjp.186.3.215. PMID 15738502.
- ↑ Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T (Jul 1994). "Effects of aging on acute toxicity of nicotine in rats". Pharmacol Toxicol. 75 (1): 1–6. doi:10.1111/j.1600-0773.1994.tb00316.x. PMID 7971729.
- ↑ Toxicology and Applied Pharmacology. Vol. 44, Pg. 1, 1978.
- ↑ "Consumer Updates: Nicotine Replacement Therapy Labels May Change". FDA. April 1, 2013.
- ↑ Lockhart LP (1933). "Nicotine poisoning". Br Med J. 1 (3762): 246–7. doi:10.1136/bmj.1.3762.246-c.
- ↑ 52.0 52.1 52.2 Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues Clin Neurosci. 15 (4): 431–443. PMC 3898681. PMID 24459410.
- ↑ 53.0 53.1 53.2 Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". Am J Drug Alcohol Abuse. 40 (6): 428–437. doi:10.3109/00952990.2014.933840. PMID 25083822.
The knowledge of DFosB induction in chronic drug exposure provides a novel method for the evaluation of substance addiction profiles (i.e. how addictive they are). Xiong et al. used this premise to evaluate the potential addictive profile of propofol (119). Propofol is a general anaesthetic, however its abuse for recreational purpose has been documented (120). Using control drugs implicated in both DFosB induction and addiction (ethanol and nicotine), ...
Conclusions
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure. The formation of ΔFosB in multiple brain regions, and the molecular pathway leading to the formation of AP-1 complexes is well understood. The establishment of a functional purpose for ΔFosB has allowed further determination as to some of the key aspects of its molecular cascades, involving effectors such as GluR2 (87,88), Cdk5 (93) and NFkB (100). Moreover, many of these molecular changes identified are now directly linked to the structural, physiological and behavioral changes observed following chronic drug exposure (60,95,97,102). New frontiers of research investigating the molecular roles of ΔFosB have been opened by epigenetic studies, and recent advances have illustrated the role of ΔFosB acting on DNA and histones, truly as a ‘‘molecular switch’’ (34). As a consequence of our improved understanding of ΔFosB in addiction, it is possible to evaluate the addictive potential of current medications (119), as well as use it as a biomarker for assessing the efficacy of therapeutic interventions (121,122,124). - ↑ Marttila K, Raattamaa H, Ahtee L (July 2006). "Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice". Neuropharmacology. 51 (1): 44–51. doi:10.1016/j.neuropharm.2006.02.014. PMID 16631212.
- ↑ Dickson, Suzanne L.; Egecioglu, Emil; Landgren, Sara; Skibicka, Karolina P.; Engel, Jörgen A.; Jerlhag, Elisabet (2011). "The role of the central ghrelin system in reward from food and chemical drugs". Molecular and Cellular Endocrinology. 340 (1): 80–7. doi:10.1016/j.mce.2011.02.017. PMID 21354264.
- ↑ Katzung, Bertram G. (2006). Basic and Clinical Pharmacology. New York: McGraw-Hill Medical. pp. 99–105.
- ↑ Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA (March 2009). "Nicotine binding to brain receptors requires a strong cation-pi interaction". Nature. 458 (7237): 534–7. Bibcode:2009Natur.458..534X. doi:10.1038/nature07768. PMC 2755585. PMID 19252481.
- ↑ 58.0 58.1 Herraiz T, Chaparro C (2005). "Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors". Biochem. Biophys. Res. Commun. 326 (2): 378–86. doi:10.1016/j.bbrc.2004.11.033. PMID 15582589.
- ↑ Fowler JS, Volkow ND, Wang GJ; et al. (1998). "Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition". J Addict Dis. 17 (1): 23–34. doi:10.1300/J069v17n01_03. PMID 9549600.
- ↑ Villégier AS, Blanc G, Glowinski J, Tassin JP (September 2003). "Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors". Pharmacology, Biochemistry, and Behavior. 76 (2): 267–74. doi:10.1016/S0091-3057(03)00223-5. PMID 14592678.
- ↑ Villégier AS, Salomon L, Granon S; et al. (August 2006). "Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine". Neuropsychopharmacology. 31 (8): 1704–13. doi:10.1038/sj.npp.1300987. PMID 16395299.
- ↑ Wüllner U, Gündisch D, Herzog H; et al. (January 2008). "Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain". Neuroscience Letters. 430 (1): 34–7. doi:10.1016/j.neulet.2007.10.011. PMID 17997038.
- ↑ Walsh H, Govind AP, Mastro R; et al. (2008). "Up-regulation of nicotinic receptors by nicotine varies with receptor subtype". J. Biol. Chem. 283 (10): 6022–32. doi:10.1074/jbc.M703432200. PMID 18174175.
- ↑ Nguyen HN, Rasmussen BA, Perry DC (2003). "Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography". J. Pharmacol. Exp. Ther. 307 (3): 1090–7. doi:10.1124/jpet.103.056408. PMID 14560040.
- ↑ Pons S, Fattore L, Cossu G; et al. (November 2008). "Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration". J. Neurosci. 28 (47): 12318–27. doi:10.1523/JNEUROSCI.3918-08.2008. PMC 2819191. PMID 19020025.
- ↑ Amir Levine; et al. (2011). "Molecular Mechanism for a Gateway Drug: Epigenetic Changes Initiated by Nicotine Prime Gene Expression by Cocaine". Sci Transl Med. 3 (107): 107ra109. doi:10.1126/scitranslmed.3003062.
- ↑ Volkow ND (November 2011). "Epigenetics of nicotine: another nail in the coughing". Sci Transl Med. 3 (107): 107ps43. doi:10.1126/scitranslmed.3003278. PMC 3492949. PMID 22049068.
- ↑ Yoshida T, Sakane N, Umekawa T, Kondo M (Jan 1994). "Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress". Physiol. Behav. 55 (1): 53–7. doi:10.1016/0031-9384(94)90009-4. PMID 8140174.
- ↑ King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET (June 2009). "Link between facultative melanin and tobacco use among African Americans". Pharmacol. Biochem. Behav. 92 (4): 589–96. doi:10.1016/j.pbb.2009.02.011. PMID 19268687.
- ↑ Elaine N. Marieb and Katja Hoehn (2007). Human Anatomy & Physiology (7th Ed.). Pearson. pp. ?. ISBN 0-8053-5909-5.[page needed]
- ↑ Bhalala, Oneil (Spring 2003). "Detection of Cotinine in Blood Plasma by HPLC MS/MS". MIT Undergraduate Research Journal. 8: 45–50.
- ↑ Le Houezec J (September 2003). "Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review". The International Journal of Tuberculosis and Lung Disease. 7 (9): 811–9. PMID 12971663.
- ↑ Benowitz NL, Jacob P, Jones RT, Rosenberg J (May 1982). "Interindividual variability in the metabolism and cardiovascular effects of nicotine in man". The Journal of Pharmacology and Experimental Therapeutics. 221 (2): 368–72. PMID 7077531.
- ↑ Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br Med J. 1980 April 5; 280(6219): 972–976.
- ↑ Hukkanen J, Jacob P, Benowitz NL (March 2005). "Metabolism and disposition kinetics of nicotine". Pharmacological Reviews. 57 (1): 79–115. doi:10.1124/pr.57.1.3. PMID 15734728.
- ↑ "The danger of third-hand smoke". Chromatography Online. 7 (3). 22 February 2011.
- ↑ Benowitz, N. L.; Herrera, B; Jacob p, 3rd (2004). "Mentholated Cigarette Smoking Inhibits Nicotine Metabolism". Journal of Pharmacology and Experimental Therapeutics. 310 (3): 1208–15. doi:10.1124/jpet.104.066902. PMID 15084646.
- ↑ www.sciencelab.com/msds.php?msdsId=9926222 Material Safety Data Sheet L-Nicotine MSDS
- ↑ Gause, G. F. (1941). "Chapter V: Analysis of various biological processes by the study of the differential action of optical isomers". In Luyet, B. J. Optical Activity and Living Matter. A series of monographs on general physiology. 2. Normandy, Missouri: Biodynamica.
- ↑ 80.0 80.1 http://library.sciencemadness.org/library/books/the_plant_alkaloids.pdf
- ↑ "Tobacco (leaf tobacco)". Transportation Information Service.
- ↑ "The Nicotine Content of Common Vegetables". The New England Journal of Medicine. 329: 437. August 1993. doi:10.1056/NEJM199308053290619.
- ↑ Lamberts, Burton L.; Dewey, Lovell J.; Byerrum, Richard U. (1959). "Ornithine as a precursor for the pyrrolidine ring of nicotine". Biochimica et Biophysica Acta. 33 (1): 22–6. doi:10.1016/0006-3002(59)90492-5. PMID 13651178.
- ↑ Dawson, R. F.; Christman, D. R.; d'Adamo, A.; Solt, M. L.; Wolf, A. P. (1960). "The Biosynthesis of Nicotine from Isotopically Labeled Nicotinic Acids1". Journal of the American Chemical Society. 82 (10): 2628–2633. doi:10.1021/ja01495a059.
- ↑ Ashihara, Hiroshi; Crozier, Alan; Komamine, Atsushi (eds.). Plant metabolism and biotechnology. Cambridge: Wiley. ISBN 978-0-470-74703-2.[page needed]
- ↑ Benowitz NL, Hukkanen J, Jacob P (2009). "Nicotine Psychopharmacology". Handbook of experimental pharmacology. Handbook of Experimental Pharmacology. 192 (192): 29–60. doi:10.1007/978-3-540-69248-5_2. ISBN 978-3-540-69246-1. PMC 2953858. PMID 19184645.
|chapter=ignored (help) - ↑ Baselt, Randall Clint (2014). Disposition of Toxic Drugs and Chemicals in Man (10th ed.). Biomedical Publications. pp. 1452–6. ISBN 978-0-9626523-9-4.
- ↑ Mündel T, Jones DA (July 2006). "Effect of transdermal nicotine administration on exercise endurance in men". Experimental Physiology. 91 (4): 705–13. doi:10.1113/expphysiol.2006.033373. PMID 16627574.
- ↑ 89.0 89.1 Rang H. P et al., Rang and Dale's Pharmacology 6th Edition, 2007, Elsevier, page 598
- ↑ US Code of Federal Regulations. 7 CFR 205.602 - Nonsynthetic substances prohibited for use in organic crop production
- ↑ Staff, IFOAM. Criticisms and Frequent Misconceptions about Organic Agriculture: The Counter-Arguments: Misconception Number 7 [dead link]
- ↑ USEPA (29 October 2008). "Nicotine; Notice of Receipt of Request to Voluntarily Cancel a Pesticide Registration". Federal Register: 64320–64322. Retrieved 8 April 2012.
- ↑ USEPA (3 June 2009). "Nicotine; Product Cancellation Order". Federal Register: 26695–26696. Retrieved 8 April 2012.
- ↑ Posselt, W.; Reimann, L. (1828). "Chemische Untersuchung des Tabaks und Darstellung eines eigenthümlich wirksamen Prinzips dieser Pflanze". Magazin für Pharmacie (in German). 6 (24): 138–161. Unknown parameter
|trans_title=ignored (help) - ↑ Henningfield JE, Zeller M (March 2006). "Nicotine psychopharmacology research contributions to United States and global tobacco regulation: a look back and a look forward". Psychopharmacology. 184 (3–4): 286–91. doi:10.1007/s00213-006-0308-4. PMID 16463054.
- ↑ Melsens, Louis-Henri-Frédéric (1843) "Note sur la nicotine," Annales de chimie et de physique, third series, vol. 9, pages 465-479; see especially page 470. [Note: The empirical formula that Melsens provides is incorrect because at that time, chemists used the wrong atomic mass for carbon (6 instead of 12).]
- ↑ Pinner, A.; Wolffenstein, R. (1891). "Ueber Nicotin". Berichte der deutschen chemischen Gesellschaft. 24: 1373–1377. doi:10.1002/cber.189102401242.
- ↑ Pinner, A. (1893). "Ueber Nicotin. Die Constitution des Alkaloïds". Berichte der deutschen chemischen Gesellschaft. 26: 292–305. doi:10.1002/cber.18930260165.
- ↑ Pinner, A. (1893). "Ueber Nicotin. I. Mitteilung". Archiv der Pharmazie. 231 (5–6): 378–448. doi:10.1002/ardp.18932310508.
- ↑ Pictet, Amé; Rotschy, A. (1904). "Synthese des Nicotins". Berichte der deutschen chemischen Gesellschaft. 37 (2): 1225–1235. doi:10.1002/cber.19040370206.
- ↑ Connolly GN, Alpert HR, Wayne GF, Koh H (October 2007). "Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997-2005". Tobacco Control. 16 (5): e5. doi:10.1136/tc.2006.019695. PMC 2598548. PMID 17897974.
- ↑ Mineur YS, Picciotto MR (December 2010). "Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis". Trends Pharmacol. Sci. 31 (12): 580–6. doi:10.1016/j.tips.2010.09.004. PMC 2991594. PMID 20965579.
- ↑ Salín-Pascual RJ1, Rosas M, Jimenez-Genchi A, Rivera-Meza BL, Delgado-Parra V (September 1996). "Antidepressant effect of transdermal nicotine patches in nonsmoking patients with major depression". J Clin Psychiatry. 59 (9): 387–9. PMID 9746444.
- ↑ Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C (2008). "Smoking, dementia and cognitive decline in the elderly, a systematic review". BMC Geriatr. 8: 36. doi:10.1186/1471-2318-8-36. PMC 2642819. PMID 19105840.
- ↑ Henningfield JE, Zeller M (2009). "Nicotine psychopharmacology: policy and regulatory". Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 192 (192): 511–34. doi:10.1007/978-3-540-69248-5_18. ISBN 978-3-540-69246-1. PMID 19184661.
- ↑ Grizzell, JA; Echeverria, V (Jun 2014). "New insights into the mechanisms of action of cotinine and its distinctive effects from nicotine". Neurochemical Research. 27. PMID 24970109.
- ↑ Crooks, PA; Dwoskin, LP (Oct 1997). "Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking". Biochem Pharmacol. 1 (54): 743–53. PMID 9353128.
- ↑ Barreto, GE; Iarkov, A; Moran, VE (Jan 2015). "Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson's disease". Front Aging Neuroscience. 9 (6): 340. doi:10.3389/fnagi.2014.00340. PMID 25620929.
External links[edit | edit source]
| Wikimedia Commons has media related to Nicotine. |
- Description of nicotine mechanisms
- Erowid Nicotine Vault : Nicotine Material Safety Data Sheet
- Thomas, Gareth AO; Rhodes, John; Ingram, John R (2005). "Mechanisms of Disease: Nicotine—a review of its actions in the context of gastrointestinal disease". Nature Clinical Practice Gastroenterology & Hepatology. 2 (11): 536–544. doi:10.1038/ncpgasthep0316.
- CDC - NIOSH Pocket Guide to Chemical Hazards
 KSF
KSF