Parkinson’s disease
 From Wikidoc - Reading time: 28 min
From Wikidoc - Reading time: 28 min
| Parkinson's disease | |
 | |
|---|---|
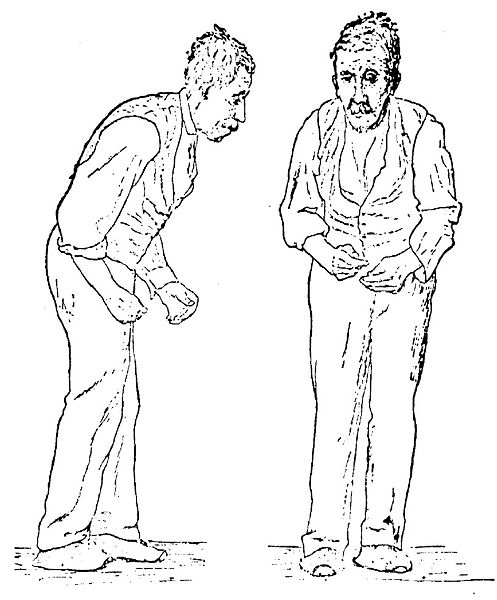
| Illustration of the Parkinson disease by Sir William Richard Gowers from A Manual of Diseases of the Nervous System in 1886 | |
| ICD-10 | G20, F02.3 |
| ICD-9 | 332 |
| DiseasesDB | 9651 |
| MedlinePlus | 000755 |
| eMedicine | neuro/304 neuro/635 in young pmr/99 rehab |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Parkinson's disease (also known as Parkinson disease or PD) is a degenerative disorder of the central nervous system that often impairs the sufferer's motor skills and speech.
Parkinson's disease belongs to a group of conditions called movement disorders. It is characterized by muscle rigidity, tremor, a slowing of physical movement (bradykinesia) and, in extreme cases, a loss of physical movement (akinesia). The primary symptoms are the results of decreased stimulation of the motor cortex by the basal ganglia, normally caused by the insufficient formation and action of dopamine, which is produced in the dopaminergic neurons of the brain. Secondary symptoms may include high level cognitive dysfunction and subtle language problems. PD is both chronic and progressive.
PD is the most common cause of parkinsonism, a group of similar symptoms. PD is also called "primary parkinsonism" or "idiopathic PD" (having no known cause). While most forms of parkinsonism are idiopathic, there are some cases where the symptoms may result from toxicity, drugs, genetic mutation, head trauma, or other medical disorders.
History[edit | edit source]
Symptoms of Parkinson's disease have been known and treated since ancient times.[1]
However, it was not formally recognized and its symptoms were not documented until 1817 in An Essay on the Shaking Palsy[2] by the British physician James Parkinson. Parkinson's disease was then known as paralysis agitans, the term "Parkinson's disease" being coined later by Jean-Martin Charcot. The underlying biochemical changes in the brain were identified in the 1950s due largely to the work of Swedish scientist Arvid Carlsson, who later went on to win a Nobel Prize. L-dopa entered clinical practice in 1967,[3] and the first study reporting improvements in patients with Parkinson's disease resulting from treatment with L-dopa was published in 1968.[4]
Symptoms[edit | edit source]
Parkinson disease affects movement (motor symptoms). Typical other symptoms include disorders of mood, behavior, thinking, and sensation (non-motor symptoms). Individual patients' symptoms may be quite dissimilar and progression of the disease is also distinctly individual.
Motor symptoms[edit | edit source]
The cardinal symptoms are:
- tremor: normally 4-7 Hz tremor, maximal when the limb is at rest, and decreased with voluntary movement. It is typically unilateral at onset. This is the most apparent and well-known symptom, though an estimated 30% of patients have little perceptible tremor; these are classified as akinetic-rigid.
- rigidity: stiffness; increased muscle tone. In combination with a resting tremor, this produces a ratchety, "cogwheel" rigidity when the limb is passively moved.
- bradykinesia/akinesia: respectively, slowness or absence of movement. Rapid, repetitive movements produce a dysrhythmic and decremental loss of amplitude. Also "dysdiadokinesia", which is the loss of ability to perform rapid alternating movements
- postural instability: failure of postural reflexes, which leads to impaired balance and falls.
Other motor symptoms include:
- Gait and posture disturbances:
- Shuffling: gait is characterized by short steps, with feet barely leaving the ground, producing an audible shuffling noise. Small obstacles tend to trip the patient
- Decreased arm swing: a form of bradykinesia
- Turning "en bloc": rather than the usual twisting of the neck and trunk and pivoting on the toes, PD patients keep their neck and trunk rigid, requiring multiple small steps to accomplish a turn.
- Stooped, forward-flexed posture. In severe forms, the head and upper shoulders may be bent at a right angle relative to the trunk (camptocormia) [5].
- Festination: a combination of stooped posture, imbalance, and short steps. It leads to a gait that gets progressively faster and faster, often ending in a fall.
- Gait freezing: "freezing" is another word for akinesia, the inability to move. Gait freezing is characterized by inability to move the feet, especially in tight, cluttered spaces or when initiating gait.
- Dystonia (in about 20% of cases): abnormal, sustained, painful twisting muscle contractions, usually affecting the foot and ankle, characterized by toe flexion and foot inversion, interfering with gait. However, dystonia can be quite generalized, involving a majority of skeletal muscles; such episodes are acutely painful and completely disabling.
- Speech and swallowing disturbances
- Hypophonia: soft speech. Speech quality tends to be soft, hoarse, and monotonous. Some people with Parkinson's disease claim that their tongue is "heavy" or have cluttered speech.[6].
- Festinating speech: excessively rapid, soft, poorly-intelligible speech.
- Drooling: most likely caused by a weak, infrequent swallow and stooped posture.
- Non-motor causes of speech/language disturbance in both expressive and receptive language: these include decreased verbal fluency and cognitive disturbance especially related to comprehension of emotional content of speech and of facial expression[7]
- Dysphagia: impaired ability to swallow. Can lead to aspiration, pneumonia.
- Other motor symptoms:
- fatigue (up to 50% of cases);
- masked faces (a mask-like face also known as hypomimia), with infrequent blinking;[8]
- difficulty rolling in bed or rising from a seated position;
- micrographia (small, cramped handwriting);
- impaired fine motor dexterity and motor coordination;
- impaired gross motor coordination;
- Poverty of movement: overall loss of accessory movements, such as decreased arm swing when walking, as well as spontaneous movement.
Non-motor symptoms[edit | edit source]
Mood disturbances[edit | edit source]
- Estimated prevalence rates of depression vary widely according to the population sampled and methodology used. Reviews of depression estimate its occurrence in anywhere from 20-80% of cases.[9] Estimates from community samples tend to find lower rates than from specialist centres. Most studies use self-report questionnaires such as the Beck Depression Inventory, which may overinflate scores due to physical symptoms. Studies using diagnostic interviews by trained psychiatrists also report lower rates of depression.
- More generally, there is an increased risk for any individual with depression to go on to develop Parkinson's disease at a later date.[10]
- 70% of individuals with Parkinson's disease diagnosed with pre-existing depression go on to develop anxiety. 90% of Parkinson's disease patients with pre-existing anxiety subsequently develop depression; apathy or abulia.
Cognitive disturbances[edit | edit source]
- slowed reaction time; both voluntary and involuntary motor responses are significantly slowed.
- executive dysfunction, characterized by difficulties in: differential allocation of attention, impulse control, set shifting, prioritizing, evaluating the salience of ambient data, interpreting social cues, and subjective time awareness. This complex is present to some degree in most Parkinson's patients; it may progress to:
- dementia: a later development in approximately 20-40% of all patients, typically starting with slowing of thought and progressing to difficulties with abstract thought, memory, and behavioral regulation. Hallucinations, delusions and paranoia may develop.
- short term memory loss; procedural memory is more impaired than declarative memory. Prompting elicits improved recall.
- medication effects: some of the above cognitive disturbances are improved by dopaminergic medications, while others are actually worsened.[11]
Sleep disturbances[edit | edit source]
- Excessive daytime somnolence
- Initial, intermediate, and terminal insomnia
- Disturbances in REM sleep: disturbingly vivid dreams, and REM Sleep Disorder, characterized by acting out of dream content - can occur years prior to diagnosis
Sensation disturbances[edit | edit source]
- impaired visual contrast sensitivity, spatial reasoning, colour discrimination, convergence insufficiency (characterized by double vision) and oculomotor control
- dizziness and fainting; usually attributable orthostatic hypotension, a failure of the autonomous nervous system to adjust blood pressure in response to changes in body position
- impaired proprioception (the awareness of bodily position in three-dimensional space)
- reduction or loss of sense of smell (microsmia or anosmia) - can occur years prior to diagnosis,
- pain: neuropathic, muscle, joints, and tendons, attributable to tension, dystonia, rigidity, joint stiffness, and injuries associated with attempts at accommodation
Autonomic disturbances[edit | edit source]
- oily skin and seborrheic dermatitis[12]
- urinary incontinence, typically in later disease progression
- nocturia (getting up in the night to pass urine) - up to 60% of cases
- constipation and gastric dysmotility that is severe enough to endanger comfort and even health
- altered sexual function: characterized by profound impairment of sexual arousal, behavior, orgasm, and drive is found in mid and late Parkinson disease. Current data addresses male sexual function almost exclusively
- weight loss, which is significant over a period of ten years - 8% of body weight lost compared with 1% in a control group.
Diagnosis[edit | edit source]

There are currently no blood or laboratory tests that have been proven to help in diagnosing PD. Therefore the diagnosis is based on medical history and a neurological examination. The disease can be difficult to diagnose accurately. The Unified Parkinson's Disease Rating Scale is the primary clinical tool used to assist in diagnosis and determine severity of PD. Indeed, only 75% of clinical diagnoses of PD are confirmed at autopsy.[13] Early signs and symptoms of PD may sometimes be dismissed as the effects of normal aging. The physician may need to observe the person for some time until it is apparent that the symptoms are consistently present. Usually doctors look for shuffling of feet and lack of swing in the arms. Doctors may sometimes request brain scans or laboratory tests in order to rule out other diseases. However, CT and MRI brain scans of people with PD usually appear normal.
Descriptive epidemiology[edit | edit source]
Parkinson's disease is widespread, with a prevalence estimated between 100 and 250 cases per 100,000 in North America; and was 1.7 per hundred (95% CI 1.5–1.9) in China (for those aged ≥65 years).[14] Because prevalence rates can be affected by socio-ecomically driven differences in survival as well as biased by survey technique problems,[15] incidence is a more sensitive indicator: rates to a high of 20.5 per 100,000 in the U.S.A. [16] A study carried out in northern California observed an age and sex corrected incidence.[17]
Cases of PD are reported at all ages, though it is uncommon in people younger than 40. The average age at which symptoms begin in the U.S.A. is 58–60; it is principally a disease of the elderly. It occurs in all parts of the world, but appears to be more common in people of European ancestry than in those of African ancestry. Those of East Asian ancestry have an intermediate risk. It is more common in rural than urban areas and men are affected more often than women in most countries.
Related diseases[edit | edit source]
There are other disorders that are called Parkinson-plus diseases. These include:
- Multiple system atrophy (MSA)
- Progressive supranuclear palsy (PSP)
- Corticobasal degeneration (CBD)
Some people include dementia with Lewy bodies (DLB) as one of the 'Parkinson-plus' syndromes. Although idiopathic Parkinson's disease patients also have Lewy bodies in their brain tissue, the distribution is denser and more widespread in DLB. Even so, the relationship between Parkinson disease, Parkinson disease with dementia (PDD) and dementia with Lewy bodies (DLB) might be most accurately conceptualized as a spectrum, with a discrete area of overlap between each of the three disorders. The natural history and role of Lewy bodies is very little understood.
Patients often begin with typical Parkinson's disease symptoms which persist for some years; these Parkinson-plus diseases can only be diagnosed when other symptoms become apparent with the passage of time. These Parkinson-plus diseases usually progress more quickly than typical ideopathic Parkinson disease. The usual anti-Parkinson's medications are typically either less effective or not effective at all in controlling symptoms; patients may be exquisitely sensitive to neuroleptic medications like haloperidol. Additionally, the cholinesterase inhibiting medications have shown preliminary efficacy in treating the cognitive, psychiatric, and behavioral aspects of the disease, so correct differential diagnosis is important.
Wilson's disease (hereditary copper accumulation) may present with parkinsonistic features; young patients presenting with parkinsonism may be screened for this rare condition. Essential tremor is often mistaken for Parkinson's disease but usually lacks all features besides tremor.
Torsion dystonia is another disease related to Parkinson's disease.
Pathology[edit | edit source]
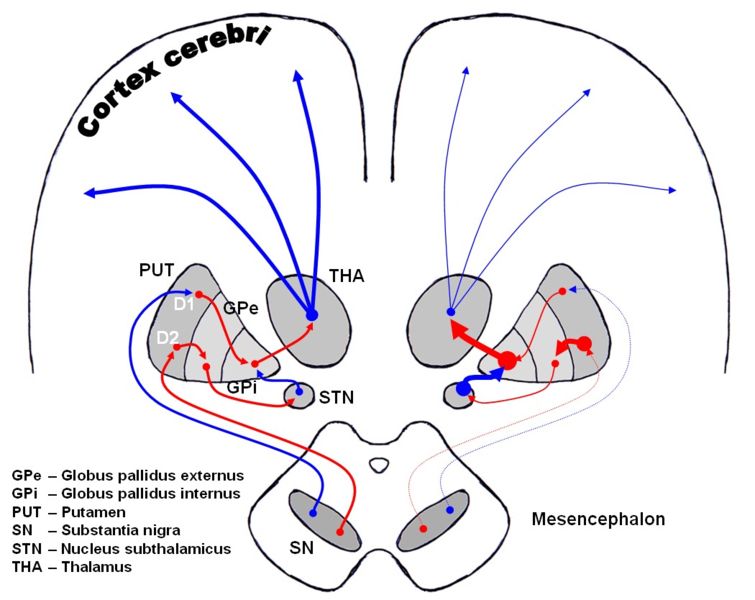
The direct pathway facilitates movement and the indirect pathway inhibits movement, thus the loss of these cells leads to a hypokinetic movement disorder. The lack of dopamine results in increased inhibition of the ventral lateral nucleus of the thalamus, which sends excitatory projections to the motor cortex, thus leading to hypokinesia.
There are four major dopamine pathways in the brain; the nigrostriatal pathway, referred to above, mediates movement and is the most conspicuously affected in early Parkinson's disease. The other pathways are the mesocortical, the mesolimbic, and the tuberoinfundibular. These pathways are associated with, respectively: volition and emotional responsiveness; desire, initiative, and reward; and sensory processes and maternal behavior. Disruption of dopamine along the non-striatal pathways likely explains much of the neuropsychiatric pathology associated with Parkinson's disease.
The mechanism by which the brain cells in Parkinson's are lost may consist of an abnormal accumulation of the protein alpha-synuclein bound to ubiquitin in the damaged cells. The alpha-synuclein-ubiquitin complex cannot be directed to the proteosome. This protein accumulation forms proteinaceous cytoplasmic inclusions called Lewy bodies. Latest research on pathogenesis of disease has shown that the death of dopaminergic neurons by alpha-synuclein is due to a defect in the machinery that transports proteins between two major cellular organelles — the endoplasmic reticulum (ER) and the Golgi apparatus. Certain proteins like Rab1 may reverse this defect caused by alpha-synuclein in animal models.[18]
Excessive accumulations of iron, which are toxic to nerve cells, are also typically observed in conjunction with the protein inclusions. Iron and other transition metals such as copper bind to neuromelanin in the affected neurons of the substantia nigra. So, neuromelanin may be acting as a protective agent. Alternately, neuromelanin (an electronically active semiconductive polymer) may play some other role in neurons.[19] That is, coincidental excessive accumulation of transition metals, etc. on neuromelanin may figure in the differential dropout of pigmented neurons in Parkinsonism. The most likely mechanism is generation of reactive oxygen species.[20]
Iron induces aggregation of synuclein by oxidative mechanisms.[21] Similarly, dopamine and the byproducts of dopamine production enhance alpha-synuclein aggregation. The precise mechanism whereby such aggregates of alpha-synuclein damage the cells is not known. The aggregates may be merely a normal reaction by the cells as part of their effort to correct a different, as-yet unknown, insult. Based on this mechanistic hypothesis, a transgenic mouse model of Parkinson's has been generated by introduction of human wild-type α-synuclein into the mouse genome under control of the platelet-derived-growth factor-β promoter.[22]
-
Dopaminergic pathways of the human brain in normal condition (left) and Parkinson's disease (right). Red Arrows indicate suppression of the target, blue arrows indicate stimulation of target structure.
The symptoms of Parkinson's disease result from the loss of pigmented dopamine-secreting (dopaminergic) cells, secreted by the same cells, in the pars compacta region of the substantia nigra (literally "black substance"). These neurons project to the striatum and their loss leads to alterations in the activity of the neural circuits within the basal ganglia that regulate movement, in essence an inhibition of the direct pathway and excitation of the indirect pathway.
Causes of Parkinson's disease[edit | edit source]
Most people with Parkinson's disease are described as having idiopathic Parkinson's disease (having no specific cause). There are far less common causes of Parkinson's disease including genetic, toxins, head trauma, and drug-induced Parkinson's disease.
Genetic[edit | edit source]
In recent years, a number of specific genetic mutations causing Parkinson's disease have been discovered, including in certain populations (Contursi, Italy). These account for a small minority of cases of Parkinson's disease. Somebody who has Parkinson's disease is more likely to have relatives that also have Parkinson's disease. However, this does not mean that the disorder has been passed on genetically.
Genetic forms that have been identified include:
- external links in this section are to OMIM
| Type | OMIM | Locus | Details |
| PARK1 | OMIM #168601 | 4q21 | caused by mutations in the SNCA gene, which codes for the protein alpha-synuclein. PARK1 causes autosomal dominant Parkinson disease. So-called PARK4 (OMIM #605543) is probably caused by triplication of SNCA.[23] |
| PARK2 | OMIM *602544 | 6q25.2-q27 | caused by mutations in protein parkin. Parkin mutations may be one of the most common known genetic causes of early-onset Parkinson disease. In one study, of patients with onset of Parkinson disease prior to age 40 (10% of all PD patients), 18% had parkin mutations, with 5% homozygous mutations.[24] Patients with an autosomal recessive family history of parkinsonism are much more likely to carry parkin mutations if age at onset is less than 20 (80% vs. 28% with onset over age 40).[25]Patients with parkin mutations (PARK2) do not have Lewy bodies. Such patients develop a syndrome that closely resembles the sporadic form of PD; however, they tend to develop symptoms at a much younger age. |
| PARK3 | OMIM %602404 | 2p13 | autosomal dominant, only described in a few kindreds. |
| PARK5 | OMIM +191342 | 4p14 | caused by mutations in the UCHL1 gene which codes for the protein ubiquitin carboxy-terminal hydrolase L1 |
| PARK6 | OMIM #605909 | 1p36 | caused by mutations in PINK1 (OMIM *608309) which codes for the protein PTEN-induced putative kinase 1. |
| PARK7 | OMIM #606324 | 1p36 | caused by mutations in DJ-1 (OMIM 602533) |
| PARK8 | OMIM #607060 | 12q12 | caused by mutations in LRRK2 which codes for the protein dardarin. In vitro, mutant LRRK2 causes protein aggregation and cell death, possibly through an interaction with parkin.[26] LRRK2 mutations, of which the most common is G2019S, cause autosomal dominant Parkinson disease, with a penetrance of nearly 100% by age 80.[27] G2019S is the most common known genetic cause of Parkinson disease, found in 1-6% of U.S. and European PD patients.[28] It is especially common in Ashkenazi Jewish patients, with a prevalence of 29.7% in familial cases and 13.3% in sporadic.[29] |
| PARK9 | OMIM #606693 | 1p36 | Caused by mutations in the ATP13A2 gene, and also known as Kufor-Rakeb Syndrome. PARK9 may be allelic to PARK6. |
| PARK10 | OMIM %606852 | 1p | - |
| PARK11 | OMIM %607688 | 2q36-37 | However, this gene locus has conflicting data, and may not have significance. |
| PARK12 | OMIM %300557 | Xq21-q25 | - |
| PARK13 | OMIM #610297 | 2p12 | Caused by mutations in the HTRA2 (HtrA serine peptidase 2) gene. |
Toxins[edit | edit source]
One theory holds that the disease may result in many or even most cases from the combination of a genetically determined vulnerability to environmental toxins along with exposure to those toxins.[30] This hypothesis is consistent with the fact that Parkinson's disease is not distributed homogeneously throughout the population: rather, its incidence varies geographically. It would appear that incidence varies by time as well, for although the later stages of untreated PD are distinct and readily recognizable, the disease was not remarked upon until the beginnings of the Industrial Revolution, and not long thereafter become a common observation in clinical practice. The toxins most strongly suspected at present are certain pesticides and transition-series metals such as manganese or iron, especially those that generate reactive oxygen species,[20][31] and or bind to neuromelanin, as originally suggested by G.C. Cotzias.[32][33]. In the Cancer Prevention Study II Nutrition Cohort, a longitudinal investigation, individuals who were exposed to pesticides had a 70% higher incidence of PD than individuals who were not exposed[34].
MPTP is used as a model for Parkinson's as it can rapidly induce parkinsonian symptoms in human beings and other animals, of any age. MPTP was notorious for a string of Parkinson's disease cases in California in 1982 when it contaminated the illicit production of the synthetic opiate MPPP. Its toxicity likely comes from generation of reactive oxygen species through tyrosine hydroxylation.[35]
Other toxin-based models employ PCBs,[36] paraquat[37] (a herbicide) in combination with maneb (a fungicide)[38] rotenone[39] (an insecticide), and specific organochlorine pesticides including dieldrin[40] and lindane.[41] Numerous studies have found an increase in PD in persons who consume rural well water; researchers theorize that water consumption is a proxy measure of pesticide exposure. In agreement with this hypothesis are studies which have found a dose-dependent an increase in PD in persons exposed to agricultural chemicals.
Head trauma[edit | edit source]
Past episodes of head trauma are reported more frequently by sufferers than by others in the population.[42][43][44] A methodologically strong recent study[42] found that those who have experienced a head injury are four times more likely to develop Parkinson’s disease than those who have never suffered a head injury. The risk of developing Parkinson’s increases eightfold for patients who have had head trauma requiring hospitalization, and it increases 11-fold for patients who have experienced severe head injury. The authors comment that since head trauma is a rare event, the contribution to PD incidence is slight. They express further concern that their results may be biased by recall, i.e., the PD patients because they reflect upon the causes of their illness, may remember head trauma better than the non-ill control subjects. These limitations were overcome recently by Tanner and colleagues,[45] who found a similar risk of 3.8, with increasing risk associated with more severe injury and hospitalization.
Drug-induced[edit | edit source]
Antipsychotics, which are used to treat schizophrenia and psychosis, can induce the symptoms of Parkinson's disease (or parkinsonism) by lowering dopaminergic activity. Due to feedback inhibition, L-dopa can also eventually cause the symptoms of Parkinson's disease that it initially relieves. Dopamine agonists can also eventually contribute to Parkinson's disease symptoms by decreasing the sensitivity of dopamine receptors.
Treatment[edit | edit source]
Parkinson's disease is a chronic disorder that requires broad-based management including patient and family education, support group services, general wellness maintenance, exercise, and nutrition. At present, there is no cure for PD, but medications or surgery can provide relief from the symptoms.
Levodopa[edit | edit source]
-
Stalevo for treatment of Parkinson's disease
The most widely used form of treatment is L-dopa in various forms. L-dopa is transformed into dopamine in the dopaminergic neurons by L-aromatic amino acid decarboxylase (often known by its former name dopa-decarboxylase). However, only 1-5% of L-DOPA enters the dopaminergic neurons. The remaining L-DOPA is often metabolised to dopamine elsewhere, causing a wide variety of side effects. Due to feedback inhibition, L-dopa results in a reduction in the endogenous formation of L-dopa, and so eventually becomes counterproductive.
Carbidopa and benserazide are dopa decarboxylase inhibitors. They help to prevent the metabolism of L-dopa before it reaches the dopaminergic neurons and are generally given as combination preparations of carbidopa/levodopa (co-careldopa) (e.g. Sinemet, Parcopa) and benserazide/levodopa (co-beneldopa) (e.g. Madopar). There are also controlled release versions of Sinemet and Madopar that spread out the effect of the L-dopa. Duodopa is a combination of levodopa and carbidopa, dispersed as a viscous gel. Using a patient-operated portable pump, the drug is continuously delivered via a tube directly into the upper small intestine, where it is rapidly absorbed.
Tolcapone inhibits the COMT enzyme, thereby prolonging the effects of L-dopa, and so has been used to complement L-dopa. However, due to its possible side effects such as liver failure, it's limited in its availability.
A similar drug, entacapone, has similar efficacy and has not been shown to cause significant alterations of liver function. A recent follow-up study by Cilia and colleagues[46] looked at the clinical effects of long-term administration of entacapone, on motor performance and pharmacological compensation, in advanced PD patients with motor fluctuations: 47 patients with advanced PD and motor fluctuations were followed for six years from the first prescription of entacapone and showed a stabilization of motor conditions, reflecting entacapone can maintain adequate inhibition of COMT over time.[46] Mucuna pruriens, is a natural source of therapeutic quantities of L-dopa, and has been under some investigation[47]
Dopamine agonists[edit | edit source]
The dopamine-agonists bromocriptine, pergolide, pramipexole, ropinirole , cabergoline, apomorphine, and lisuride, are moderately effective. These have their own side effects including those listed above in addition to somnolence, hallucinations and /or insomnia. Several forms of dopamine agonism have been linked with a markedly increased risk of problem gambling. Dopamine agonists initially act by stimulating some of the dopamine receptors. However, they cause the dopamine receptors to become progressively less sensitive, thereby eventually increasing the symptoms.
Dopamine agonists can be useful for patients experiencing on-off fluctuations and dyskinesias as a result of high doses of L-dopa. Apomorphine can be administered via subcutaneous injection using a small pump which is carried by the patient. A low dose is automatically administered throughout the day, reducing the fluctuations of motor symptoms by providing a steady dose of dopaminergic stimulation. After an initial "apomorphine challenge" in hospital to test its effectiveness and brief patient and caregiver, the primary caregiver (often a spouse or partner) takes over maintenance of the pump. The injection site must be changed daily and rotated around the body to avoid the formation of nodules. Apomorphine is also available in a more acute dose as an autoinjector pen for emergency doses such as after a fall or first thing in the morning.
MAO-B inhibitors[edit | edit source]
Selegiline and rasagiline reduce the symptoms by inhibiting monoamine oxidase-B (MAO-B), which inhibits the breakdown of dopamine secreted by the dopaminergic neurons. Metabolites of selegiline include L-amphetamine and L-methamphetamine (not to be confused with the more notorious and potent dextrorotary isomers). This might result in side effects such as insomnia. Use of L-dopa in conjunction with selegiline has increased mortality rates that have not been effectively explained. Another side effect of the combination can be stomatitis. One report raised concern about increased mortality when MAO-B inhibitors were combined with L-dopa;[48] however subsequent studies have not confirmed this finding.[49] Unlike other non selective monoamine oxidase inhibitors, tyramine-containing foods do not cause a hypertensive crisis.
Speech therapies[edit | edit source]
The most widely practiced treatment for the speech disorders associated with Parkinson's disease is Lee Silverman Voice Treatment (LSVT). LSVT focuses on increasing vocal loudness.[50]
A study found that an electronic device providing frequency-shifted auditory feedback (FAF) improved the clarity of Parkinson's patients' speech.[51]
Physical exercise[edit | edit source]
Regular physical exercise and/or therapy, including in forms such as yoga, tai chi, and dance can be beneficial to the patient for maintaining and improving mobility, flexibility, balance and a range of motion. Physicians and physical therapists often recommend basic exercises, such as bringing the toes up with every step, carrying a bag with weight to decrease the bend having on one side, and practicing chewing hard and move the food around the mouth.[52]
Surgery and deep brain stimulation[edit | edit source]

Treating Parkinson's disease with surgery was once a common practice, but after the discovery of levodopa, surgery was restricted to only a few cases. Studies in the past few decades have led to great improvements in surgical techniques, and surgery is again being used in people with advanced PD for whom drug therapy is no longer sufficient.
Deep brain stimulation is presently the most used surgical means of treatment, but other surgical therapies that have shown promise include surgical lesion of the subthalamic nucleus[53] and of the internal segment of the globus pallidus, a procedure known as pallidotomy.[54]
Methods undergoing evaluation[edit | edit source]
Gene therapy[edit | edit source]
Currently under investigation is gene therapy. This involves using a harmless virus to shuttle a gene into a part of the brain called the subthalamic nucleus (STN). The gene used leads to the production of an enzyme called glutamic acid decarboxylase (GAD), which catalyses the production of a neurotransmitter called GABA.[55] GABA acts as a direct inhibitor on the overactive cells in the STN.
GDNF infusion involves the infusion of GDNF (glial-derived neurotrophic factor) into the basal ganglia using surgically implanted catheters. Via a series of biochemical reactions, GDNF stimulates the formation of L-dopa. GDNF therapy is still in development.
Implantation of stem cells genetically engineered to produce dopamine or stem cells that transform into dopamine-producing cells has already started being used. These could not constitute cures because they do not address the considerable loss of activity of the dopaminergic neurons. Initial results have been unsatifactory, with patients still retaining their drugs and symptoms.
Neuroprotective treatments[edit | edit source]
Neuroprotective treatments are at the forefront of PD research, but are still under clinical scrutiny[56]. These agents could protect neurons from cell death induced by disease presence resulting in a slower pregression of disease. Agents currently under investigation as neuroprotective agents include apoptotic drugs (CEP 1347 and CTCT346), lazaroids, bioenergetics, antiglutamatergic agents and dopamine receptors[57]. Clinically evaluated neuroprotective agents are the monoamine oxidase inhibitors selegiline[58] and rasagiline, dopamine agonists, and the complex I mitochondrial fortifier coenzyme Q10.
Neural transplantation[edit | edit source]
The first prospective randomised double-blind sham-placebo controlled trial of dopamine-producing cell transplants failed to show an improvement in quality of life although some significant clinical improvements were seen in patients below the age of 60.[59] A significant problem was the excess release of dopamine by the transplanted tissue, leading to dystonias.[60] Research in African green monkeys suggests that the use of stem cells might in future provide a similar benefit without inducing dystonias.[61]
Nutrients[edit | edit source]
Nutrients have been used in clinical studies and are widely used by people with Parkinson's disease in order to partially treat PD or slow down its deterioration. The L-dopa precursor L-tyrosine was shown to relieve an average of 70% of symptoms.[62] Ferrous iron, the essential cofactor for L-dopa biosynthesis was shown to relieve between 10% and 60% of symptoms in 110 out of 110 patients.[63] [64]
More limited efficacy has been obtained with the use of THFA, NADH, and pyridoxine—coenzymes and coenzyme precursors involved in dopamine biosynthesis.[65] Vitamin C and vitamin E in large doses are commonly used by patients in order to theoretically lessen the cell damage that occurs in Parkinson's disease. This is because the enzymes superoxide dismutase and catalase require these vitamins in order to nullify the superoxide anion, a toxin commonly produced in damaged cells. However, in the randomized controlled trial, DATATOP of patients with early PD, no beneficial effect for vitamin E compared to placebo was seen.[58]
Coenzyme Q10 has more recently been used for similar reasons. MitoQ is a newly developed synthetic substance that is similar in structure and function to coenzyme Q10.
Qigong[edit | edit source]
There have been two studies looking at qigong in Parkinson's disease. In a trial in Bonn, an open-label randomised pilot study in 56 patients found an improvement in motor and non-motor symptoms amongst patients who had undergone one hour of structured Qigong exercise per week in two 8-week blocks. The authors speculate that visualizing the flow of "energy" might act as an internal cue and so help improve movement.[66]
The second study, however, found Qigong to be ineffective in treating Parkinson's disease. In that study, researchers used a randomized cross-over trial to compare aerobic training with Qigong in advanced Parkinson's disease. Two groups of PD patients were assessed, had 20 sessions of either aerobic exercise or qigong, were assessed again, then after a 2 month gap were switched over for another 20 sessions, and finally assessed again. The authors found an improvement in motor ability and cardiorespiratory function following aerobic exercise, but found no benefit following Qigong. The authors also point out that aerobic exercise had no benefit for patients' quality of life.[67]
Botox[edit | edit source]
Recently, Botox injections are being investigated as a non-FDA approved possible experimental treatment.[68]
Prognosis[edit | edit source]
PD is not considered to be a fatal disease by itself, but it progresses with time. The average life expectancy of a PD patient is generally lower than for people who do not have the disease.[69] In the late stages of the disease, PD may cause complications such as choking, pneumonia, and falls that can lead to death.
The progression of symptoms in PD may take 20 years or more. In some people, however, the disease progresses more quickly. There is no way to predict what course the disease will take for an individual person. With appropriate treatment, most people with PD can live productive lives for many years after diagnosis.
In at least some studies, it has been observed that mortality was significantly increased, and longevity decreased among nursing home patients as compared to community dwelling patients.
One commonly used system for describing how the symptoms of PD progress is called the Hoehn and Yahr scale. Another commonly used scale is the Unified Parkinson's Disease Rating Scale (UPDRS). This much more complicated scale has multiple ratings that measure motor function, and also mental functioning, behavior, mood, and activities of daily living; and motor function. Both the Hoehn and Yahr scale and the UPDRS are used to measure how individuals are faring and how much treatments are helping them. It should be noted that neither scale is specific to Parkinson's disease; that patients with other illnesses can score in the Parkinson's range.
Notable Parkinson's sufferers[edit | edit source]
One famous sufferer of young-onset Parkinson's is Michael J. Fox, whose book, Lucky Man (2000), focused on his experiences with the disease and his career and family travails in the midst of it. Fox established The Michael J. Fox Foundation for Parkinson's Research to develop a cure for Parkinson's disease within this decade.
Other famous sufferers include Pope John Paul II, playwright Eugene O'Neill, artist Salvador Dalí, evangelist Billy Graham, former US Attorney General Janet Reno, and boxer Muhammad Ali. Political figures suffering from it have included Adolf Hitler, Francisco Franco, Deng Xiaoping and Mao Zedong, and former Prime Minister of Canada Pierre Trudeau. Numerous actors have also been afflicted with Parkinson's such as: Terry-Thomas, Deborah Kerr, Kenneth More, Vincent Price, Jim Backus and Michael Redgrave. Helen Beardsley (of Yours, Mine and Ours fame) also suffered from this disease toward the end of her life. Director George Roy Hill (The Sting, Butch Cassidy and the Sundance Kid) also suffered from Parkinson's disease.
The film Awakenings (starring Robin Williams and Robert De Niro and based on genuine cases reported by Oliver Sacks) deals sensitively and largely accurately with a similar disease, postencephalitic parkinsonism
References[edit | edit source]
- This article contains text released into the public domain from:
National Institute of Neurological Disorders and Stroke (January 2006). "Parkinson's Disease: Hope Through Research". National Institutes of Health.
- ↑ Manyam BV, Sánchez-Ramos JR (1999). "Traditional and complementary therapies in Parkinson's disease". Advances in neurology. 80: 565–74. PMID 10410773.
- ↑ Parkinson J (2002). "An essay on the shaking palsy. 1817" (Reproduced\). J Neuropsychiatry Clin Neurosci. 14 (2): 223–36, discussion 222. PMID 11983801.
- ↑ Hornykiewicz O (2002). "L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent". Amino Acids. 23 (1–3): 65–70. doi:10.1007/s00726-001-0111-9. PMID 12373520.
- ↑ Cotzias, G. (1968). "L-Dopa for Parkinsonism". N Engl J Med. 278 (11): 630. PMID 5637779.
- ↑ Lepoutre A, Devos D, Blanchard-Dauphin A; et al. (2006). "A specific clinical pattern of camptocormia in Parkinson's disease". J. Neurol. Neurosurg. Psychiatr. 77 (11): 1229–34. PMID 16735399.
- ↑ Fox, Michael (2003). Lucky Man: A Memoir. Hyperion. p. 214. ISBN 0786888741. Unknown parameter
|middle=ignored (help) - ↑ Pell M (1996). "On the receptive prosodic loss in Parkinson's disease". Cortex. 32 (4): 693–704. PMID 8954247.
- ↑ Deuschl G, Goddemeier C (1998). "Spontaneous and reflex activity of facial muscles in dystonia, Parkinson's disease, and in normal subjects". J. Neurol. Neurosurg. Psychiatr. 64 (3): 320–4. PMID 9527141.
- ↑ Lieberman A (2006). "Depression in Parkinson's disease -- a review". Acta Neurol Scand. 113 (1): 1–8. PMID 16367891.
- ↑ Ishihara L, Brayne C (2006). "A systematic review of depression and mental illness preceding Parkinson's disease". Acta Neurol Scand. 113 (4): 211–20. PMID 16542159.
- ↑ Frank MJ (2005). "Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism". Journal of cognitive neuroscience. 17 (1): 51–72. doi:10.1162/0898929052880093. PMID 15701239.
- ↑ Gupta A, Bluhm R (2004). "Seborrheic dermatitis". Journal of the European Academy of Dermatology and Venereology : JEADV. 18 (1): 13–26, quiz 19-20. PMID 14678527.
- ↑ Gelb D, Oliver E, Gilman S (1999). "Diagnostic criteria for Parkinson disease". Arch Neurol. 56 (1): 33–9. PMID 9923759.
- ↑ Zhang ZX, Roman GC, Hong Z; et al. (2005). "Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai". Lancet. 365 (9459): 595&ndash, 7. doi:10.1016/S0140-6736(05)17909-4. PMID 15708103.
- ↑ Bermejo F, Gabriel R, Vega S, Morales JM, Rocca WA, Anderson DW (2001). "Problems and issues with door-to-door, two-phase surveys: an illustration from central Spain". Neuroepidemiology. 20 (4): 225&ndash, 31. PMID 11684897.
- ↑ Rajput (1984). "Epidemiology of Parkinson's disease". Can J Neurol Sci. 11 (1): 156&ndash, 159. PMID 6713314.
- ↑ Van Den Eeden S, Tanner C, Bernstein A, Fross R, Leimpeter A, Bloch D, Nelson L (2003). "Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity". Am J Epidemiol. 157 (11): 1015&ndash, 22. PMID 12777365.
- ↑ "Parkinson's Disease Mechanism Discovered," HHMI Research News June 22, 2006.
- ↑ McGinness J, Corry P, Proctor P (1974). "Amorphous semiconductor switching in melanins" (Reprint). Science. 183 (127): 853–5. PMID 4359339.
- ↑ 20.0 20.1 Jenner P (1998). "Oxidative mechanisms in nigral cell death in Parkinson's disease". Mov Disord. 13 Suppl 1: 24–34. PMID 9613715.
- ↑ Kaur D, Andersen J (2002). "Ironing out Parkinson's disease: is therapeutic treatment with iron chelators a real possibility?" (PDF). Aging Cell. 1 (1): 17–21. PMID 12882349.
- ↑ Masliah E, Rockenstein E, Veinbergs I; et al. (2000). "Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders". Science. 287 (5456): 1265–9. PMID 10678833.
- ↑ Singleton AB, Farrer M, Johnson J; et al. (2003). "alpha-Synuclein locus triplication causes Parkinson's disease". Science. 302 (5646): 841. doi:10.1126/science.1090278. PMID 14593171.
- ↑ Poorkaj P; et al. (2004). "parkin mutation analysis in clinic patients with early-onset Parkinson's disease". American Journal of Medical Genetics Part A. 129A (1): 44&ndash, 50.
- ↑ Ebba Lohmann; et al. (2003). "How much phenotypic variation can be attributed to parkin genotype?". Annals of Neurology. 54 (2): 176&ndash, 185. PMID 12891670.
- ↑ Smith WW; et al. (2005). "Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration". Proceedings of the National Academy of Sciences of the United States of America. 102 (51): 18676&ndash, 18681. PMID 16352719.
- ↑ Kachergus J, Mata IF, Hulihan M; et al. (2005). "Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations". Am. J. Hum. Genet. 76 (4): 672–80. doi:10.1086/429256. PMID 15726496.
- ↑ Brice A (2005). "Genetics of Parkinson's disease: LRRK2 on the rise". Brain. 128 (Pt 12): 2760–2. doi:10.1093/brain/awh676. PMID 16311269.
- ↑ Ozelius L, Senthil G, Saunders-Pullman R; et al. (2006). "LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews". N Engl J Med. 354 (4): 424–5. PMID 16436782.
- ↑ Di Monte DA, Lavasani M, Manning-Bog AB (2002). "Environmental factors in Parkinson's disease". Neurotoxicology. 23 (4–5): 487–502. PMID 12428721.
- ↑ Chiueh CC, Andoh T, Lai AR, Lai E, Krishna G (2000). "Neuroprotective strategies in Parkinson's disease: protection against progressive nigral damage induced by free radicals". Neurotoxicity research. 2 (2–3): 293–310. PMID 16787846.
- ↑ Cotzias G (1966). "Manganese, melanins and the extrapyramidal system". J Neurosurg. 24 (1): Suppl:170-80. PMID 4955707.
- ↑ Barbeau A (1984). "Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias)". Neurotoxicology. 5 (1): 13–35. PMID 6538948.
- ↑ Ascherio A, Chen H, Weisskopf M; et al. (2006). "Pesticide exposure and risk for Parkinson's disease". Ann Neurol. 60 (2): 197–203. PMID 16802290.
- ↑ Chiueh C, Wu R, Mohanakumar K, Sternberger L, Krishna G, Obata T, Murphy D (1994). "In vivo generation of hydroxyl radicals and MPTP-induced dopaminergic toxicity in the basal ganglia". Ann N Y Acad Sci. 738: 25–36. PMID 7832434.
- ↑ Orr, Leslie (February 10, 2005). "PCBs, fungicide open brain cells to Parkinson's assault". Medical News Today.
- ↑ Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA (2002). "The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein". J. Biol. Chem. 277 (3): 1641–4. doi:10.1074/jbc.C100560200. PMID 11707429.
- ↑ Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA (2000). "The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease". J. Neurosci. 20 (24): 9207–14. PMID 11124998.
- ↑ Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000). "Chronic systemic pesticide exposure reproduces features of Parkinson's disease". Nat. Neurosci. 3 (12): 1301–6. doi:10.1038/81834. PMID 11100151.
- ↑ Kitazawa M, Anantharam V, Kanthasamy AG (2001). "Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells". Free Radic. Biol. Med. 31 (11): 1473–85. PMID 11728820.
- ↑ Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D (2000). "Organochlorine insecticides in substantia nigra in Parkinson's disease". J. Toxicol. Environ. Health Part A. 59 (4): 229–34. PMID 10706031.
- ↑ 42.0 42.1 Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA (2003). "Head trauma preceding PD: a case-control study". Neurology. 60 (10): 1610–5. PMID 12771250.
- ↑ Stern M, Dulaney E, Gruber SB; et al. (1991). "The epidemiology of Parkinson's disease. A case-control study of young-onset and old-onset patients". Arch. Neurol. 48 (9): 903–7. PMID url=http://archneur.ama-assn.org/cgi/content/abstract/48/9/903 1953412 url=http://archneur.ama-assn.org/cgi/content/abstract/48/9/903 Check
|pmid=value (help). - ↑ Uryu K, Giasson BI, Longhi L; et al. (2003). "Age-dependent synuclein pathology following traumatic brain injury in mice". Exp. Neurol. 184 (1): 214–24. PMID 14637093.
- ↑ Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW (2006). "Head injury and Parkinson's disease risk in twins". Ann. Neurol. 60 (1): 65–72. doi:10.1002/ana.20882. PMID 16718702.
- ↑ 46.0 46.1
R. Cilia; et al. (2006). "Long-term Efficacy of Entacapone in Patients with Parkinson's Disease and Motor Fluctuations - A Six-Year Clinical Follow-Up Study". line feed character in
|title=at position 50 (help) - ↑ Katzenschlager R, Evans A, Manson A; et al. (2004). "Mucuna pruriens in Parkinson's disease: a double blind clinical and pharmacological study". J. Neurol. Neurosurg. Psychiatr. 75 (12): 1672–7. doi:10.1136/jnnp.2003.028761. PMID 15548480.
- ↑ Thorogood M, Armstrong B, Nichols T, Hollowell J (1998). "Mortality in people taking selegiline: observational study". BMJ. 317 (7153): 252–4. PMID 9677215.
- ↑ Marras C, McDermott M, Rochon P, Tanner C, Naglie G, Rudolph A, Lang A (2005). "Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort". Neurology. 64 (1): 87–93. PMID 15642909.
- ↑ "What is LSVT?" http://www.lsvt.org/main_site.htm
- ↑ Lowit, A., Brendel, B. "The response of patients with Parkinson's Disease to DAF and FSF," Stammering Research April 2004.
- ↑ Garg, R and Lakhan, S. Parkinson's Disease - Pharmaceutical and Physical Therapies. GNIF Brain Blogger. August, 2006.
- ↑ Guridi J, Obeso JA (2001). "The subthalamic nucleus, hemiballismus and Parkinson's disease: reappraisal of a neurosurgical dogma". Brain. 124 (Pt 1): 5–19. PMID 11133783.
- ↑ Fukuda M, Kameyama S, Yoshino M, Tanaka R, Narabayashi H (2000). "Neuropsychological outcome following pallidotomy and thalamotomy for Parkinson's disease". Stereotactic and functional neurosurgery. 74 (1): 11–20. PMID 11124660.
- ↑ Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ (2007). "Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial". Lancet. 369 (9579): 2097–105. doi:10.1016/S0140-6736(07)60982-9. PMID 17586305.
- ↑ Bonuccelli U,
Del Dotto P (2006). "New pharmacologic horizons in the treatment of Parkinson disease". Neurology. 67 (2): 30–38. line feed character in
|author=at position 14 (help) - ↑ Djaldetti R, Melamed E (2002). "New drugs in the future treatment of Parkinson's disease". J. Neurol. 249 Suppl 2: II30–5. doi:10.1007/s00415-002-1206-2. PMID 12375061.
- ↑ 58.0 58.1 "Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group". N. Engl. J. Med. 328 (3): 176–83. 1993. PMID 8417384.
- ↑ Freed CR, Greene PE, Breeze RE; et al. (2001). "Transplantation of embryonic dopamine neurons for severe Parkinson's disease". N. Engl. J. Med. 344 (10): 710–9. PMID 11236774.
- ↑ Redmond DE (2002). "Cellular replacement therapy for Parkinson's disease--where we are today?". The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 8 (5): 457–88. PMID 12374430.
- ↑ Redmond E; et al. (2007). "Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells". Procedings of the National Academy of Sciences. 104 (29).
- ↑ Lemoine P, Robelin N, Sebert P, Mouret J (1986). "La L-tyrosine : traitement au long cours de la maladie de Parkinson [L-tyrosine : A long term treatment of Parkinson's Disease]". Comptes rendus academie des sciences (in French). 309: 43–47.
- ↑ Birkmayer W, Birkmayer JG (1986). "Iron, a new aid in the treatment of Parkinson patients". J. Neural Transm. 67 (3–4): 287–92. PMID 3806082.
- ↑ Editors Przuntek H , Riederer P, ed. (1989). Early diagnosis and preventive therapy in Parkinson's disease. Springer. pp. p. 323. ISBN 0-387-82080-9.
- ↑ "Dopamine biosynthesis" (Word doc). University of Chicago Personal Web Pages. Retrieved 2006-11-04.
- ↑ Schmitz-Hubsch T (2006). "Qigong exercise for the symptoms of Parkinson's disease: a randomized, controlled pilot study". Mov Disord. 21 (4): 543–548. PMID 16229022.
- ↑ Burini D, Farabollini B, Iacucci S; et al. (2006). "A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson's disease". Europa medicophysica. 42 (3): 231–8. PMID 17039221.
- ↑ Template:Citeref patent
- ↑ "Parkinson's Disease". Mayo Clinic: College of Medicine. Retrieved 2006-11-04.
External links[edit | edit source]
| Wikimedia Commons has media related to Parkinson's disease. |
- National and International Organisations
- American Parkinson Disease Association
- European Parkinson's Disease Association
- Northwest Parkinson's Foundation (US)
- National Parkinson Foundation (US)
- Parkinson Society Canada
- Parkinson's Disease Society (UK)
- Parkinson's Disease Foundation (US)
- Parkinson's Action Network (US)
- Parkinsons Research Foundation (US)
- World Parkinson Disease Association
- Hope For A Cure Foundation for Parkinson's Research
- Charts
- Other sites
Template:Diseases of the nervous system
ar:مرض باركنسن zh-min-nan:Parkinson ê pēⁿ ca:Malaltia de Parkinson cs:Parkinsonova choroba da:Parkinsons sygdom de:Parkinson-Krankheit et:Parkinsoni tõbi el:Νόσος του Πάρκινσον eo:Parkinsono ko:파킨슨씨 병 hr:Parkinsonova bolest id:Penyakit Parkinson it:Morbo di Parkinson ku:Nexweşiya parkinsonê he:מחלת פרקינסון lb:Parkinson-Krankheet ms:Penyakit Parkinson nl:Ziekte van Parkinson no:Parkinsons sykdom fi:Parkinsonin tauti sv:Parkinsons sjukdom th:โรคพาร์กินสัน
 KSF
KSF