Positron emission tomography
 From Wikidoc - Reading time: 14 min
From Wikidoc - Reading time: 14 min
Editor-in-Chief: Thomas F. Heston, MD, PhD
Overview[edit | edit source]
Positron emission tomography (PET) is a nuclear medicine medical imaging technique which produces a three-dimensional image or map of functional processes in the body. The concept of emission and transmission tomography was introduced by David Kuhl and Roy Edwards in the late 1950's. Their work later led to the design and construction of several tomographic instruments at the University of Pennsylvania. The technique was further developed by Michel (Michael) Ter-Pogossian, Michael E. Phelps and others at the Washington University School of Medicine [1][2]
Description[edit | edit source]
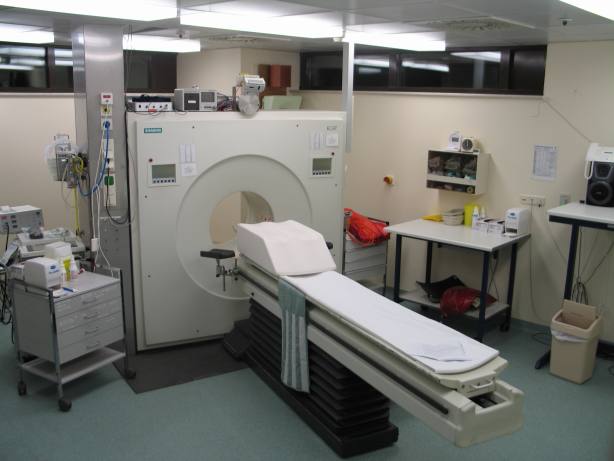
How a PET scan is conducted[edit | edit source]
To conduct the scan, a short-lived radioactive tracer isotope, which decays by emitting a positron, which also has been chemically incorporated into a metabolically active molecule, is injected into the living subject (usually into blood circulation). There is a waiting period while the metabolically active molecule becomes concentrated in tissues of interest; then the research subject or patient is placed in the imaging scanner. The molecule most commonly used for this purpose is fluorodeoxyglucose (FDG), a sugar, for which the waiting period is typically an hour.
How the scanner operates[edit | edit source]
As the radioisotope undergoes positron emission decay (also known as positive beta decay), it emits a positron, the antimatter counterpart of an electron. After travelling up to a few millimeters the positron encounters and annihilates with an electron, producing a pair of annihilation (gamma) photons moving in almost opposite directions. These are detected when they reach a scintillator material in the scanning device, creating a burst of light which is detected by photomultiplier tubes or silicon avalanche photodiodes (Si APD). The technique depends on simultaneous or coincident detection of the pair of photons; photons which do not arrive in pairs (i.e., within a few nanoseconds) are ignored.
The most significant fraction of electron-positron decays result in two 511 keV gamma photons being emitted at almost 180 degrees to each other; hence it is possible to localize their source along a straight line of coincidence (also called formally the "line of response" or LOR). In practice the LOR has a finite width as the emitted photons are not exactly 180 degrees apart. If the recovery time of detectors is in the picosecond range rather than the 10's of nanosecond range, it is possible to calculate the single point on the LOR at which an annihilation event originated, by measuring the "time of flight" of the two photons. This technology is not yet common, but it is available on some new systems [1]. More commonly, a technique much like the reconstruction of computed tomography (CT) and single photon emission computed tomography (SPECT) data is used, although the data set collected in PET is much poorer than CT, so reconstruction techniques are more difficult (see section below on image reconstruction of PET). Using statistics collected from tens-of-thousands of coincidence events, a set of simultaneous equations for the total activity of each parcel of tissue along many LORs can be solved by a number of techniques, and thus a map of radioactivities as a function of location for parcels or bits of tissue ("voxels"), may be constructed and plotted. The resulting map shows the tissues in which the molecular probe has become concentrated, and can be interpreted by a nuclear medicine physician or radiologist in the context of the patient's diagnosis and treatment plan.
-
Schematic view of a detector block and ring of a PET scanner (here: Siemens ECAT Exact HR+)
-
Schema of a PET acquisition process
PET scans are increasingly read alongside CT or magnetic resonance imaging (MRI) scans, the combination (co-registration") giving both anatomic and metabolic information (i.e., what the structure is, and what it is doing biochemically). Because PET imaging is most useful in combination with anatomical imaging, such as CT, modern PET scanners are now available with integrated high-end multi-detector-row CT scanners. Because the two scans can be performed in immediate sequence during the same session, with the patient not changing position between the two types of scans, the two sets of images are more-precisely registered, so that areas of abnormality on the PET imaging can be more perfectly correlated with anatomy on the CT images. This is very useful in showing detailed views of moving organs or structures with higher amounts of anatomical variation, such as are more likely to occur outside the brain.
How PET scanning is used[edit | edit source]
PET is both a medical and research tool. It is used heavily in clinical oncology (medical imaging of tumors and the search for metastases), and for clinical diagnosis of certain diffuse brain diseases such as those causing various types of dementias. PET is also an important research tool to map normal human brain and heart function.
PET is also used in pre-clinical studies using animals, where it allows repeated investigations into the same subjects. This is particularly valuable in cancer research, as it results in an increase in the statistical quality of the data (subjects can act as their own control) and substantially reduces the numbers of animals required for a given study.
Alternative methods of scanning include x-ray computed tomography (CT), magnetic resonance imaging (MRI) and functional magnetic resonance imaging (fMRI), ultrasound and single photon emission computed tomography (SPECT).
While some imaging scans such as CT and MRI isolate organic anatomic changes in the body, PET scanners, like SPECT are capable of detecting areas of molecular biology detail (even prior to anatomic change). The PET scanner does this via the use of radiolabelled molecular probes that have different rates of uptake, depending on the type and function of tissue involved. The changing of regional blood flow in various anatomic structures (as a measure of the injected positron emitter) can be visualized and relatively quantified with a PET scan.
PET imaging is best performed using a dedicated PET scanner. However, it is possible to acquire PET images using a conventional dual-head gamma camera fitted with a coincidence detector. The quality of gamma-camera PET is considerably lower, and acquisition is slower. However, for institutions with low demand for PET, this may allow on-site imaging, instead of referring patients to another center, or relying on a visit by a mobile scanner.
-
Maximum intensity projection (MIP) of a typical F-18 FDG wholebody PET acquisition
-
PET scan of the human brain.
Radioisotopes used in PET[edit | edit source]
Radionuclides used in PET scanning are typically isotopes with short half lives such as carbon-11 (~20 min), nitrogen-13 (~10 min), oxygen-15 (~2 min), and fluorine-18 (~110 min). Due to their short half lives, the radionuclides must be produced in a cyclotron which is not too far away in delivery-time to the PET scanner. These radionuclides are incorporated into compounds normally used by the body such as glucose, water or ammonia and then injected into the body to trace where they become distributed. Such labelled compounds are known as radiotracers.
Limitations of PET[edit | edit source]
PET as a technique for scientific investigation in humans is limited by the need for clearance by ethics committees to inject radioactive material into participants. The minimization of radiation dose to the subject is an attractive feature of the use of short-lived radionuclides. Besides its established role as a diagnostic technique, PET has an expanding role as a method to assess the response to therapy, in particular, cancer therapy (e.g. Young et al. 1999), where the risk to the patient from lack of knowledge about disease progress is much greater than the risk from the test radiation.
Limitations to the widespread use of PET arise from the high costs of cyclotrons needed to produce the short-lived radionuclides for PET scanning and the need for specially adapted on-site chemical synthesis apparatus to produce the radiopharmaceuticals. Few hospitals and universities are capable of maintaining such systems, and most clinical PET is supported by third-party suppliers of radiotracers which can supply many sites simultaneously. This limitation restricts clinical PET primarily to the use of tracers labelled with F-18, which has a half life of 110 minutes and can be transported a reasonable distance before use, or to rubidium-82, which can be created in a portable generator and is used for myocardial perfusion studies. Nevertheless, in recent years a few on-site cyclotrons with integrated shielding and hot labs have begun to accompany PET units to remote hospitals. The presence of the small on-site cyclotron promises to expand in the future as the cyclotrons shrink in response to the high cost of isotope transportation to remote PET machines [3]
Because the half-life of F-18 is about two hours, the prepared dose of a radiopharmaceutical bearing this radionuclide will undergo multiple half-lives of decay during the working day. This necessitates frequent recalibration of the remaining dose (determination of activity per unit volume) and careful planning with respect to patient scheduling.
Image reconstruction in PET[edit | edit source]
The raw data collected by a PET scanner are a list of 'coincidence events' representing near-simultaneous detection of annihilation photons by a pair of detectors. Each coincidence event represents a line in space connecting the two detectors along which the positron emission occurred.
Coincidence events can be grouped into projections images, called sinograms. The sinograms are sorted by the angle of each view and tilt, the latter in 3D case images. The sinogram images are analogous to the projections captured by computed tomography (CT) scanners, and can be reconstructed in a similar way. However, the statistics of the data is much worse than those obtained through transmission tomography. A normal PET data set has millions of counts for the whole acquisition, while the CT can reach a few billion counts. Furthermore, PET data suffer from scatter and random events much more dramatically than CT data does.
In practice, considerable pre-processing of the data is required - correction for random coincidences, estimation and subtraction of scattered photons, detector dead-time correction (after the detection of a photon, the detector must "cool down" again) and detector-sensitivity correction (for both inherent detector sensitivity and changes in sensitivity due to angle of incidence).
Filtered back projection (FBP) has been frequently used to reconstruct images from the projections. This algorithm has the advantage of being simple while having a low requirement for computing resources. However, shot noise in the raw data is prominent in the reconstructed images and areas of high tracer uptake tend to form streaks across the image.
Iterative expectation-maximization algorithms are now the preferred method of reconstruction. The advantage is a better noise profile and resistance to the streak artifacts common with FBP, but the disadvantage is higher computer resource requirements.
Attenuation correction: As different LORs must traverse different thicknesses of tissue, the photons are attenuated differentially. The result is that structures deep in the body are reconstructed as having falsely low tracer uptake. Contemporary scanners can estimate attenuation using integrated x-ray CT equipment, however earlier equipment offered a crude form of CT using a gamma ray (positron emitting) source and the PET detectors.
While attenuation corrected images are generally more faithful representations, the correction process is itself susceptible to significant artifacts. As a result, both corrected and uncorrected images are always reconstructed and read together.
2D/3D reconstruction: Early PET scanners had only a single ring of detectors, hence the acquisition of data and subsequent reconstruction was restricted to a single transverse plane. More modern scanners now include multiple rings, essentially forming a cylinder of detectors.
There are two approaches to reconstructing data from such a scanner: 1) treat each ring as a separate entity, so that only coincidences within a ring are detected, the image from each ring can then be reconstructed individually (2D reconstruction), or 2) allow coincidences to be detected between rings as well as within rings, then reconstruct the entire volume together (3D).
3D techniques have better sensitivity (because more coincidences are detected and used) and therefore less noise, but are more sensitive to the effects of scatter and random coincidences, as well as requiring correspondingly greater computer resources.
More detailed applications list[edit | edit source]
PET is a valuable technique for some diseases and disorders, because it is possible to target the radio-chemicals used for particular bodily functions.
- Oncology: PET scanning with the tracer fluorine-18 (F-18) fluorodeoxyglucose (FDG), called FDG-PET, is widely used in clinical oncology. This tracer is a glucose analog that is taken up by glucose-using cells and phosphorylated by hexokinase (whose mitochondrial form is greatly elevated in rapidly-growing malignant tumours). A typical dose of FDG used in an oncological scan is 200-400 MBq for an adult human. Because the oxygen atom which is replaced by F-18 to generate FDG is required for the next step in glucose metabolism in all cells, no further reactions occur in FDG. Furthermore, most tissues (with the notable exception of liver and kidneys) cannot remove the phosphate added by hexokinase. This means that FDG is trapped in any cell which takes it up, until it decays, since phosphorylated sugars, due to their ionic charge, cannot exit from the cell. This results in intense radiolabeling of tissues with high glucose uptake, such as the brain, the liver, and most cancers. As a result, FDG-PET can be used for diagnosis, staging, and monitoring treatment of cancers, particularly in Hodgkin's disease, non Hodgkin's lymphoma, and lung cancer. Many other types of solid tumors will be found to be very highly labeled on a case-by-case basis-- a fact which becomes especially useful in searching for tumor metastasis, or for recurrence after a known highly-active primary tumor is removed. Because individual PET scans are more expensive than "conventional" imaging with computed tomography (CT) and magnetic resonance imaging (MRI), expansion of FDG-PET in cost-constrained health services will depend on proper health technology assessment; this problem is a difficult one because structural and functional imaging often cannot be directly compared, as they provide different information. Oncology scans using FDG make up over 90% of all PET scans in current practice.
- Neurology: PET neuroimaging is based on an assumption that areas of high radioactivity are associated with brain activity. What is actually measured indirectly is the flow of blood to different parts of the brain, which is generally believed to be correlated, and has been measured using the tracer oxygen-15. However, because of its 2-minute half-life O-15 must be piped directly from a medical cyclotron for such uses, and this is difficult. In practice, since the brain is normally a rapid user of glucose, and since brain pathologies such as Alzheimer's disease greatly decrease brain metabolism of both glucose and oxygen in tandem, standard FDG-PET of the brain, which measures regional glucose use, may also be successfully used to differentiate Alzheimer's disease from other dementing processes, and also to make early diagnosis of Alzheimer's disease. The advantage of FDG-PET for these uses is its much wider availability.
Several radiotracers (i.e. radioligands) have been developed for PET that are ligands for specific neuroreceptor subtypes (e.g. dopamine D2, serotonin 5-HT1A, etc.), transporters (such as [(11)C]McN5652, [(11)C]DASB or other novel tracer ligands for serotonin in this case), or enzyme substrates (e.g. 6-FDOPA for the AADC enzyme). These agents permit the visualization of neuroreceptor pools in the context of a plurality of neuropsychiatric and neurologic illnesses. A novel probe developed at the University of Pittsburgh termed PIB (Pittsburgh Compound-B) permits the visualization of amyloid plaques in the brains of Alzheimer's patients. This technology could assist clinicians in making a positive clinical diagnosis of AD pre-mortem and aid in the development of novel anti-amyloid therapies.
- Cardiology, atherosclerosis and vascular disease study: In clinical cardiology, FDG-PET can identify so-called "hibernating myocardium", but its cost-effectiveness in this role versus SPECT is unclear. Recently, a role has been suggested for FDG-PET imaging of atherosclerosis to detect patients at risk of stroke [2].
- Neuropsychology / Cognitive neuroscience: To examine links between specific psychological processes or disorders and brain activity.
- Psychiatry: Numerous compounds that bind selectively to neuroreceptors of interest in biological psychiatry have been radiolabeled with C-11 or F-18. Radioligands that bind to dopamine receptors (D1,D2, reuptake transporter), serotonin receptors (5HT1A, 5HT2A, reuptake transporter) opioid receptors (mu) and other sites have been used successfully in studies with human subjects. Studies have been performed examining the state of these receptors in patients compared to healthy controls in schizophrenia, substance abuse, mood disorders and other psychiatric conditions.
- Pharmacology: In pre-clinical trials, it is possible to radiolabel a new drug and inject it into animals. The uptake of the drug, the tissues in which it concentrates, and its eventual elimination, can be monitored far more quickly and cost effectively than the older technique of killing and dissecting the animals to discover the same information. PET scanners for rats and apes are marketed for this purpose. The technique is still generally too expensive for the veterinary medicine market, however, so very few pet PET scans are done. Drug occupancy at the purported site of action can also be inferred indirectly by competition studies between unlabeled drug and radiolabeled compounds known apriori to bind with specificity to the site.
PET scan safety[edit | edit source]
PET scanning is non-invasive, but it does involve exposure to ionizing radiation. The total dose of radiation is small, however, usually around 7 mSv. This can be compared to 2.2 mSv average annual background radiation in the UK, 0.02 mSv for a chest x-ray, up to 8 mSv for a CT scan of the chest, 2-6 mSv per annum for aircrew (data from UK National Radiological Protection Board).
Availability[edit | edit source]
In Canada, availability varies from province to province [3]. In Ontario, PET scan costs are not covered under the government-funded public health system (OHIP), but scans are available from a few public and private facilities at a cost equivalent of approximately $2500 USD. [4]
See also[edit | edit source]
References[edit | edit source]
- ↑ Simon Cherry, et al. Physics in Nuclear Medicine. Saunders Publishing. 2003. p.3
- ↑ Phelps ME, Hoffman EJ, Mullani NA, Ter-Pogossian MM. “Application of annihilation coincidence detection to transaxial reconstruction tomography.” Journal of Nuclear Medicine. 1975; 16(3):210-24.
- ↑ http://www.medicalimagingmag.com/issues/articles/2003-07_05.asp
Additional Resources[edit | edit source]
- Young H, Baum R, Cremerius U; et al. (1999). "Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations". European Journal of Cancer. 35 (13): 1773–1782.
- Bustamante E. and Pedersen P.L. (1977). "High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase". Proceedings of the National Academy of Sciences USA. 74 (9): 3735–3739.
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, and Langstrom B. (2004). "Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B". Annals of Neurology. 55 (3): 306–319.
da:Positronemissionstomografi de:Positronen-Emissions-Tomographie eo:Pozitrona Emisia Tomografio fa:پت اسکن gl:PET is:PET-skanni it:Tomografia ad emissione di positroni he:PET hu:Pozitron emissziós tomográfia nl:Positronemissietomografie no:Positronemisjonstomografi fi:Positroniemissiotomografia sv:Positronemissionstomografi
 KSF
KSF


