Pulmonary embolism CT
 From Wikidoc - Reading time: 11 min
From Wikidoc - Reading time: 11 min
| Resident Survival Guide |
|
Pulmonary Embolism Microchapters |
|
Diagnosis |
|---|
|
Pulmonary Embolism Assessment of Probability of Subsequent VTE and Risk Scores |
|
Treatment |
|
Follow-Up |
|
Special Scenario |
|
Trials |
|
Case Studies |
|
Pulmonary embolism CT On the Web |
Editor(s)-In-Chief: The APEX Trial Investigators, C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview[edit | edit source]
Pulmonary angiography is the gold standard for diagnosing a pulmonary embolism (PE). The pulmonary angiogram has a sensitivity and specificity of >95% in diagnosing a PE. Pulmonary angiography is presently used less frequently in the diagnosis of pulmonary embolism due to wider acceptance of CT scans, which are non-invasive. CT pulmonary angiography (CTPA) is the recommended first line diagnostic imaging test in most people. A negative CT pulmonary angiogram excludes a clinically important pulmonary embolism.[1] Multi-Detector Computed Tomography (MDCT) has rapidly replaced the use of pulmonary angiography in the clinical setting because MDCT is less invasive and easier to perform. Therefore, pulmonary angiography should only be performed first if MDCTA is unavailable or contraindicated.
CT Pulmonary Angiography[edit | edit source]
- Spiral CT pulmonary angiogram is the standard modality to non-invasively diagnose a pulmonary embolism.[2][3]
- Initial studies reported sensitivities for diagnosing emboli to the segmental level (4th order branch) to be as high as 98%, however subsequent studies have found sensitivities to be lower.
- The sensitivity is higher when the clot has a more proximal location.
- Although smaller clots in the subsegmental arteries are not as physiologically relevant as the larger more proximal clots, they may serve as important predictors of future, larger clots.
- A study consisting of 142 patients concluded that the sensitivity and specificity of CT angiography is higher than that of a V/Q scan.[4]
- Obtaining a CT angiography is recommended following an indeterminate V/Q scan. If the pre-test probability is ‘sufficiently high’, and CT angiography is negative, a standard CT angiogram should then be obtained.
- A cost-effective analysis using spiral CT angiography for the diagnosis of PE showed the following results.[5]
- The use of CT angiography in a diagnostic algorithm was the most cost-effective strategy.
- If the sensitivity of CT angiography was < 85%, conventional angiography was associated with a lower mortality, but still remained a more expensive strategy.
- According to the International Commission on Radiological Protection (ICRP) the radiation exposure from a V/Q scan with Tc-99 m macroaggregate of albumi (MAA) is 1.1 mSv.
- The radiation exposure from spiral CT is 2–6 mSv.[6]
- The radiation exposure from plain chest X-ray is approximately 0.05 mSv.
- Evidence of right ventricular dysfunction on CT scan demonstrated by the presence of a value of less than 1.0 for right-to-left ventricular diameter was reported to have a 100% negative predictive value for an uneventful outcome among patients with PE(95% CI: 94.3%, 100%).[7]
Diagnostic Accuracy[edit | edit source]
The diagnostic accuracy of CT pulmonary angiogram for the detection of pulmonary embolism has been reported in a meta-analysis as the following:[8]
- Sensitivity: 86%
- Specificity : 94%
Subsequently, the PIOPED II study provided similar results. PIOPED II used a mixture of 4 slice and 16 slice scanners and reported a sensitivity of 83% and a specificity of 96%.[9]
In high risk patients, a negative CT pulmonary angiogram was found be a meta-analysis to have a 8% risk of pulmonary embolism among high risk patients (defined as pre-test probability of at least 40%)[10].
Advantages[edit | edit source]
- Non-invasive nature
- Greater availability to patients
- Capable of picking up other lung disorders from the differential diagnosis in case there is no pulmonary embolism.
Limitations[edit | edit source]
- Requires reader expertise
- Non-portable and expensive
- Needs contrast bolus comparable to angiogram
- Contraindicated in renal insufficiency and in patients with contrast allergies.
CT Findings in Acute PE[edit | edit source]
- Thrombus is located centrally within the vascular lumen or occludes the vessel (vessel cut-off sign)
- Commonly causes distention of the involved vessel
CT Findings in Chronic PE[edit | edit source]
- Eccentric and contiguous changes of the vessel wall
- Reduces the arterial diameter by more than 50%
- Evidence of recanalization within the thrombus
- An arterial web is present
Single-Detector vs Multi-Detector CT[edit | edit source]
Single-Detector CT[edit | edit source]
Recent improvements in CT technology have reduced the value of CT angiography for the initial workup of PE patients. Studies surveying single detector spiral CT use in cases of suspected pulmonary embolism show wide variations in both sensitivity (53-100%) and specificity (73-100%) for detecting a PE.[11][12]
Two large multicentric clinical studies for single-detector CT, including more than 1000 patients, reported a sensitivity of 70% and a specificity of 90% for the diagnosis of a PE.[13][14] Due to motion artifacts and insufficient opacification, the rate of technical inadequacy of single detector CT in this study was 5-8%.
Two large studies have shown that a combination of a negative single detector CT and an absence of proximal lower limb DVT on lower limb venous ultrasonoagraphy in non-high clinical probability patients were associated with a 3-month thromboembolic risk of 1%.[15][16]
Multi-Detector CT[edit | edit source]
Since its introduction, CT angiography has been the method of choice for visualizing the pulmonary vasculature for suspected PE patients. Although CT angiography remains the gold standard in diagnosing a PE, MDCT and SDCT are often the initial modes of evaluating patients with a suspected PE. In comparison to angiography, a CT is less invasive, takes less time, is easier to perform, and exposes the patient to lower amounts of radiation.
Advantage of MDCT over SDCT[edit | edit source]
- High spatial resolution.
- High temporal resolution.
- Better quality of arterial opacification.
- Adequate visualization of pulmonary arteries up to at least the segmental level.
Supportive Trial Data[edit | edit source]
A study enrolling 94 patients, done in 2004, showed the sensitivity and specificity of multi-detector CT to be above 90% in the diagnosis of pulmonary embolism.[17]
The PIOPED II study, which enrolled 824 patients, published their results in 2006 showing a sensitivity and specificity of multi-detector CT to be 83% and 96% respectively in the diagnosis of PE.[9] The PIOPED II study also highlighted the influence of clinical probability on the predictive value of MDCT.
Another study with enrollment of 1819 patients, compared two diagnostic strategies based on D-dimer and MDCT, one with and the other without lower limb compression ultrasonography (CUS). The study reported that the 3-month thromboembolic risk was 0.3% (95% CI 0.1-1.1) in the D-dimer-Ultrasonography-CT (DD-US-CT) group and 0.3% (0.1-1.2) in the DD-CT group (difference 0.0% [-0.9 to 0.8]). In the DD-US-CT group, ultrasonography showed a deep-venous thrombosis in 53 (9% [7-12]) of 574 patients, and thus MDCT was not undertaken.[18]
Role of MDCT in Diagnosing PE[19][edit | edit source]
- In patients with a low or intermediate clinical probability of PE, a negative MDCT is adequate criteria for excluding PE.
- In patients with high a clinical probability of PE, and a negative CT, there is still some disagreement as to whether there should be further investigation by compression ultrasonography, ventilation-perfusion (V/Q) scan, or pulmonary angiography.[19]
- In patients with intermediate or high clinical probability of PE:
- A MDCT showing a PE at a segmental or more proximal level is adequate proof of PE in those patients.
- According to PIOPED II, the PPV of MDCT was found to be low (58%),[9] so further testing should be considered in the case of a negative MDCT.
Role of MDCT in Assessment of Right Ventricular Dysfunction[edit | edit source]
Right ventricular dysfunction is an independent predictor of clinical deterioration and death in pulmonary embolism patients, therefore it can be used for risk stratification for adverse outcomes. Thus MDCT has the potential to provide both diagnostic and prognostic stratification in acute pulmonary embolism patients.
- In a study, a right-to-left ventricular dimensional ratio of less than 0.9 on MDCT was found to have a 100% NPV for death due to PE.[20]
- This could also be used to identify those patients at a low risk of death who are candidates for early discharge or home treatment.
Isolated Subsegmental PE: Role of MDCT in Deciding Treatment[edit | edit source]
Presence of a single subsegmental clot on MDCT is termed as an isolated subsegmental PE. 1-5 % of patients with suspected PE undergoing MDCT have found to have an isolated subsegmental PE.[21][22][23]
- Positive predictable values of such findings are low.
- Compression ultrasonography is advised to rule out DVT, and it is used to assist in treating patients with isolated subsegmental PE.
- In patients with isolated subsegmental PE, but without DVT, no recommendation is made due to a lack of evidence.[19]
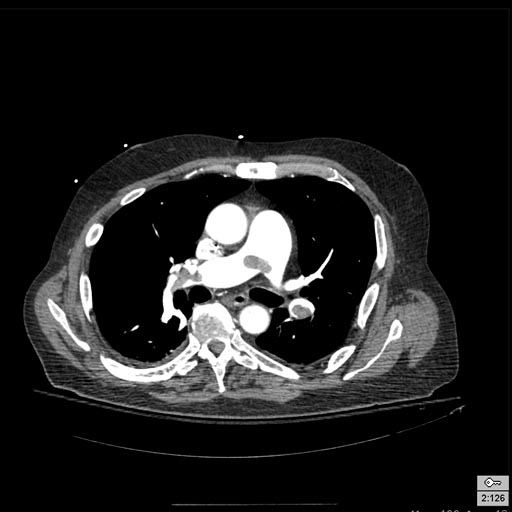
Examples of CT Images in Pulmonary Embolism[edit | edit source]
Pulmonary Septic Emboli[edit | edit source]
Septic emboli are seen most commonly in:
- Patients with infective endocarditis
- Patients with infected venous catheters or pacemaker leads
- Patients with periodontal disease
Examples of CT Images in Septic Embolism[edit | edit source]
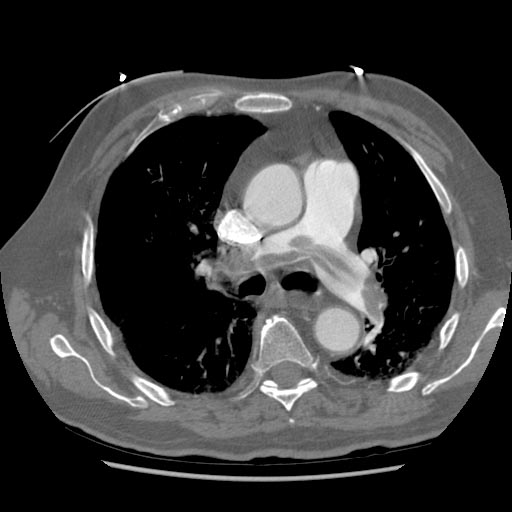
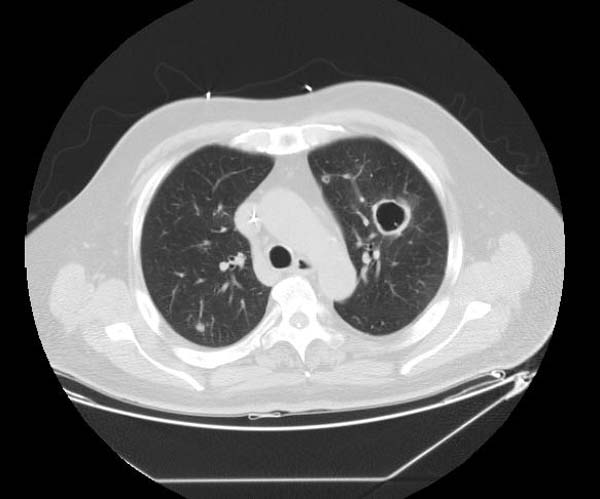
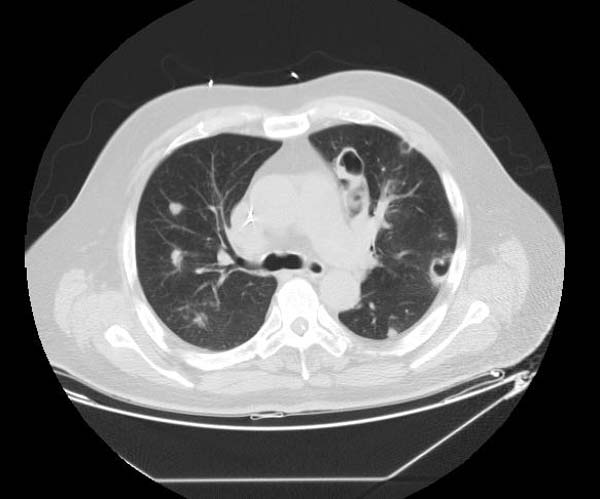
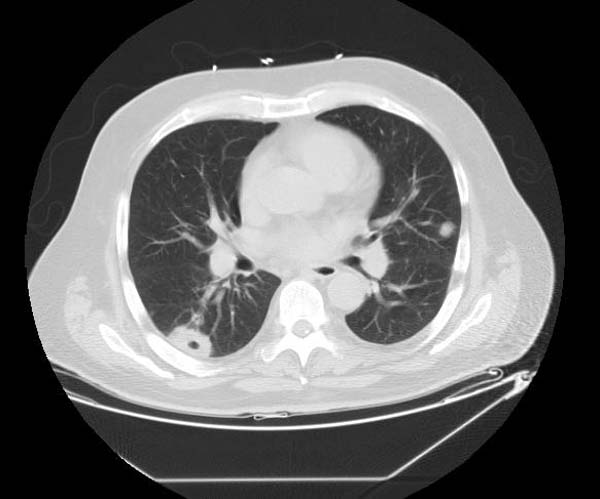
- The CT appearance of septic emboli includes nodules, wedge-shaped subpleural opacities with or without cavitation, and the feeding vessel sign.
- The feeding vessel sign consists of a distinct vessel leading directly into the center of a nodule. This sign has been considered highly suggestive of septic embolism, and the prevalence varying from 67-100% in various series (note: the feeding vessel sign also occurs in pulmonary metastasis).
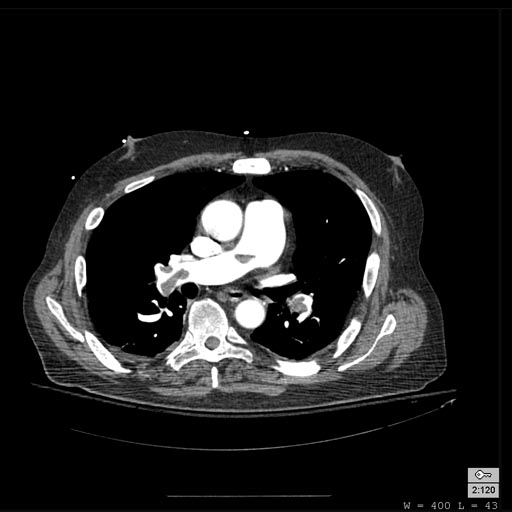
-
Pulmonary septic emboli
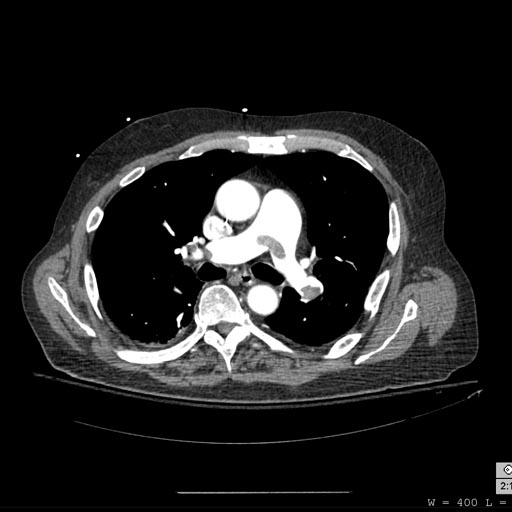
-
Pulmonary septic emboli
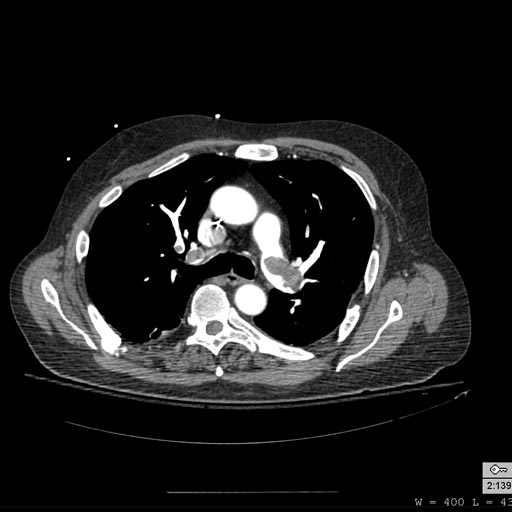
-
Pulmonary septic emboli
ESC 2008 Guideline Recommendations (DO NOT EDIT)[19][edit | edit source]
Suspected High-Risk PE Patients (DO NOT EDIT)[19][edit | edit source]
| Class I |
| 1. In high-risk pulmonary embolism, as indicated by the presence of shock or hypotension, emergency CT or bedside echocardiography (depending on availability and clinical circumstances) is recommended for diagnostic purposes. (Level of Evidence: C) |
Suspected Non High-risk PE Patients (DO NOT EDIT)[19][edit | edit source]
Low Clinical Probability[edit | edit source]
| Class I |
| 1. Normal D-dimer level using either a highly or moderately sensitive assay excludes pulmonary embolism. (Level of Evidence: A) |
Intermediate Clinical Probability[edit | edit source]
| Class I |
| 1. Negative multi-detector CT (MDCT) safely excludes pulmonary embolism. (Level of Evidence: A) |
| 2. Negative single-detector CT (SDCT) only excludes pulmonary embolism when combined with negative proximal compression ultrasonography. (Level of Evidence: A) |
| 3. SDCT or MDCT showing a segmental or more proximal thrombus confirms pulmonary embolism. (Level of Evidence: A) |
| Class IIa |
| 1. Further testing should be considered to confirm pulmonary embolsim if SDCT or MDCT shows only subsegmental clots. (Level of Evidence: B) |
High Clinical Probability[edit | edit source]
| Class I |
| 1. SDCT or MDCT showing a segmental or more proximal thrombus confirms pulmonary embolism. (Level of Evidence: A) |
| Class IIa |
| 1. In patients with a negative CT, further tests should be considered in selected patients to exclude pulmonary embolism. (Level of Evidence: B) |
References[edit | edit source]
- ↑ Stein PD, Henry JW, Gottschalk A (1999). "Reassessment of pulmonary angiography for the diagnosis of pulmonary embolism: relation of interpreter agreement to the order of the involved pulmonary arterial branch". Radiology. 210 (3): 689–91. PMID 10207468.
- ↑ Authors/Task Force Members. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D; et al. (2014). "2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS)". Eur Heart J. 35 (43): 3033–73. doi:10.1093/eurheartj/ehu283. PMID 25173341.
- ↑ Schoepf UJ, Goldhaber SZ, Costello P (2004). "Spiral computed tomography for acute pulmonary embolism". Circulation. 109 (18): 2160–7. doi:10.1161/01.CIR.0000128813.04325.08. PMID 15136509. Retrieved 2011-12-05. Unknown parameter
|month=ignored (help) - ↑ Mayo JR, Remy-Jardin M, Müller NL, Remy J, Worsley DF, Hossein-Foucher C, Kwong JS, Brown MJ (1997). "Pulmonary embolism: prospective comparison of spiral CT with ventilation-perfusion scintigraphy". Radiology. 205 (2): 447–52. PMID 9356627. Retrieved 2011-12-06. Unknown parameter
|month=ignored (help) - ↑ van Erkel AR, van Rossum AB, Bloem JL, Kievit J, Pattynama PM (1996). "Spiral CT angiography for suspected pulmonary embolism: a cost-effectiveness analysis". Radiology. 201 (1): 29–36. PMID 8816516. Retrieved 2011-12-05. Unknown parameter
|month=ignored (help) - ↑ "Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53)". Ann ICRP. 28 (3): 1–126. 1998. PMID 10840563. Retrieved 2011-12-06.
- ↑ van der Meer RW, Pattynama PM, van Strijen MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H; et al. (2005). "Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism". Radiology. 235 (3): 798–803. doi:10.1148/radiol.2353040593. PMID 15845793.
- ↑ Hayashino Y, Goto M, Noguchi Y, Fukui T (2005). "Ventilation-perfusion scanning and helical CT in suspected pulmonary embolism: meta-analysis of diagnostic performance". Radiology. 234 (3): 740–8. doi:10.1148/radiol.2343031009. PMID 15734930. Review in: ACP J Club. 2005 Sep-Oct;143(2):52
- ↑ 9.0 9.1 9.2 Stein PD, Fowler SE, Goodman LR; et al. (2006). "Multidetector computed tomography for acute pulmonary embolism". N. Engl. J. Med. 354 (22): 2317–27. doi:10.1056/NEJMoa052367. PMID 16738268.
- ↑ Belzile D, Jacquet S, Bertoletti L, Lacasse Y, Lambert C, Lega JC; et al. (2018). "Outcomes following a negative computed tomography pulmonary angiography according to pulmonary embolism prevalence: a meta-analysis of the management outcome studies". J Thromb Haemost. 16 (6): 1107–1120. doi:10.1111/jth.14021. PMID 29645405.
- ↑ Mullins MD, Becker DM, Hagspiel KD, Philbrick JT (2000). "The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism". Arch. Intern. Med. 160 (3): 293–8. PMID 10668830. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Rathbun SW, Raskob GE, Whitsett TL (2000). "Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review". Ann. Intern. Med. 132 (3): 227–32. PMID 10651604. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Perrier A, Howarth N, Didier D, Loubeyre P, Unger PF, de Moerloose P, Slosman D, Junod A, Bounameaux H (2001). "Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism". Ann. Intern. Med. 135 (2): 88–97. PMID 11453707. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Van Strijen MJ, De Monye W, Kieft GJ, Pattynama PM, Prins MH, Huisman MV (2005). "Accuracy of single-detector spiral CT in the diagnosis of pulmonary embolism: a prospective multicenter cohort study of consecutive patients with abnormal perfusion scintigraphy". J. Thromb. Haemost. 3 (1): 17–25. doi:10.1111/j.1538-7836.2004.01064.x. PMID 15634261. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Musset D, Parent F, Meyer G, Maître S, Girard P, Leroyer C, Revel MP, Carette MF, Laurent M, Charbonnier B, Laurent F, Mal H, Nonent M, Lancar R, Grenier P, Simonneau G (2002). "Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study". Lancet. 360 (9349): 1914–20. doi:10.1016/S0140-6736(02)11914-3. PMID 12493257. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Perrier A, Roy PM, Aujesky D, Chagnon I, Howarth N, Gourdier AL, Leftheriotis G, Barghouth G, Cornuz J, Hayoz D, Bounameaux H (2004). "Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study". Am. J. Med. 116 (5): 291–9. doi:10.1016/j.amjmed.2003.09.041. PMID 14984813. Retrieved 2012-04-30. Unknown parameter
|month=ignored (help) - ↑ Winer-Muram HT, Rydberg J, Johnson MS, Tarver RD, Williams MD, Shah H, Namyslowski J, Conces D, Jennings SG, Ying J, Trerotola SO, Kopecky KK (2004). "Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography". Radiology. 233 (3): 806–15. doi:10.1148/radiol.2333031744. PMID 15564410. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ Righini M, Le Gal G, Aujesky D, Roy PM, Sanchez O, Verschuren F, Rutschmann O, Nonent M, Cornuz J, Thys F, Le Manach CP, Revel MP, Poletti PA, Meyer G, Mottier D, Perneger T, Bounameaux H, Perrier A (2008). "Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non-inferiority trial". Lancet. 371 (9621): 1343–52. doi:10.1016/S0140-6736(08)60594-2. PMID 18424324. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ 19.0 19.1 19.2 19.3 19.4 19.5 Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Mayer E, Remy-Jardin M, Bassand JP (2008). "Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)". Eur. Heart J. 29 (18): 2276–315. doi:10.1093/eurheartj/ehn310. PMID 18757870. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ Becattini C, Agnelli G, Vedovati MC, Pruszczyk P, Casazza F, Grifoni S, Salvi A, Bianchi M, Douma R, Konstantinides S, Lankeit M, Duranti M (2011). "Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test". Eur. Heart J. 32 (13): 1657–63. doi:10.1093/eurheartj/ehr108. PMID 21504936. Retrieved 2012-05-01. Unknown parameter
|month=ignored (help) - ↑ Perrier A, Roy PM, Sanchez O, Le Gal G, Meyer G, Gourdier AL; et al. (2005). "Multidetector-row computed tomography in suspected pulmonary embolism". N Engl J Med. 352 (17): 1760–8. doi:10.1056/NEJMoa042905. PMID 15858185. Review in: J Fam Pract. 2005 Aug;54(8):653, 657
- ↑ Brunot S, Corneloup O, Latrabe V, Montaudon M, Laurent F (2005). "Reproducibility of multi-detector spiral computed tomography in detection of sub-segmental acute pulmonary embolism". Eur Radiol. 15 (10): 2057–63. doi:10.1007/s00330-005-2844-4. PMID 16021452.
- ↑ Eyer BA, Goodman LR, Washington L (2005). "Clinicians' response to radiologists' reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT". AJR Am J Roentgenol. 184 (2): 623–8. PMID 15671388.
 KSF
KSF