Radiography
 From Wikidoc - Reading time: 5 min
From Wikidoc - Reading time: 5 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Assistant Editor-In-Chief: Anand Patel, MD [2]
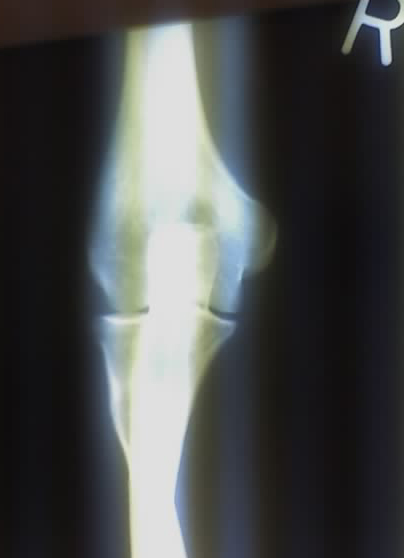
Radiography is the use of certain types of electromagnetic radiation—usually ionizing—to view objects. The use of non-ionizing radiations (visible light and ultraviolet light) to view objects should be considered as a normal “optical” method (e.g., light microscopy). The modification of an object through the use of ionizing radiation is not radiography. Depending on the nature of the object and the intended outcome it can be radiotherapy, food irradiation, or radiation processing. [3]
Medical and industrial radiography[edit | edit source]
Radiography is used for both medical and industrial applications; for further details please see the Medical radiography and Industrial radiography pages. If the object being examined is living (human or animal) it is regarded as medical, and all other radiography is regarded as being industrial radiographic work.
History of radiography[edit | edit source]
Radiography started in 1895 with the discovery of X-rays (also called Roentgen rays after the man who first described their properties in rigorous detail), a type of electromagnetic radiation. Soon X-rays were used in various applications, from helping to fit shoes, to the medical uses that have persisted. X-rays were put to diagnostic use very early, before the dangers of ionizing radiation were discovered. Initially, many kinds of staff conducted radiography in hospitals, including physicists, photographers, doctors, nurses, and engineers. The medical specialty of radiology grew up over many years around the new technology. When new diagnostic tests involving X-rays were developed, it was natural for the radiographers to be trained in and to adopt this new technology. This happened first with fluoroscopy, computed tomography (1960s), mammography, ultrasound (1970s), and magnetic resonance imaging (1980s). Although a nonspecialist dictionary might define radiography quite narrowly as "taking X-ray images", this has long been only part of the work of "X-ray departments", radiographers, and radiologists.
Equipment[edit | edit source]
Below is a very short overview. For more details please see the radiographic equipment page.
Sources[edit | edit source]
A number of sources of X-ray photons have been used, these include sealed X-ray tubes, betatrons and linacs. For gamma photons, radioactive sources such as 192Ir have been used.
Detectors[edit | edit source]
A range of detectors including photographic film, scintillator and semiconductor diode arrays have been used to collect images.
Theory of X-ray attenuation[edit | edit source]
Medical usage X-ray photons are more likely to be formed by an event involving an electron, while gamma ray photons are more likely to be formed from the nucleus of an atom. In general, medical radiography is done using X-rays formed in an X-ray tube. Nuclear medicine typically involves gamma rays.
The types of electromagnetic radiation of most interest to radiography are X-ray and gamma radiation. This radiation is much more energetic than the more familiar types such as radio waves and visible light. It is this relatively high energy which makes gamma rays useful in radiography but potentially hazardous to living organisms.
The radiation is produced by X-ray tubes, high energy X-ray equipment or natural radioactive elements, such as radium and radon, and artificially produced radioactive isotopes of elements, such as cobalt-60 and iridium-192. Electromagnetic radiation consists of oscillating electric and magnetic fields, but is generally depicted as a single sinusoidal wave. While in the past radium and radon have both been used for radiography, they have fallen out of use as they are radiotoxic alpha radiation emitters which are expensive; iridium-192 and cobalt-60 are far better photon sources. For further details see commonly used gamma emitting isotopes.
Such a wave is characterised by its wavelength (the distance from a point on one cycle to the corresponding point on the next cycle) or its frequency (the number of oscillations per second). In a vacuum, all electromagnetic waves travel at the same speed, the speed of light (c). The wavelength (λ, lambda) and the frequency (f) are all related by the equation:
- f = c / λ
This is true for all electromagnetic radiation.
Electromagnetic radiation is known by various names, depending on its energy. The energy of these waves is related to the frequency and the wavelength by the relationship:
- E = hf = h (c / λ)
Where h is a constant known as Planck's Constant.
Gamma rays are indirectly ionizing radiation. A gamma ray passes through matter until it undergoes an interaction with an atomic particle, usually an electron. During this interaction, energy is transferred from the gamma ray to the electron, which is a directly ionizing particle. As a result of this energy transfer, the electron is liberated from the atom and proceeds to ionize matter by colliding with other electrons along its path. Other times, the passing gamma ray interferes with the orbit of the electron, and slows it, releasing energy but not becoming dislodged. The atom is not ionised, and the gamma ray continues on, although at a lower energy. This energy released is usually heat or another, weaker photon, and causes biological harm as a radiation burn. The chain reaction caused by the initial dose of radiation can continue after exposure, much like a sunburn continues to damage skin even after one is out of direct sunlight.
For the range of energies commonly used in radiography, the interaction between gamma rays and electrons occurs in two ways. One effect takes place where all the gamma ray's energy is transmitted to an entire atom. The gamma ray no longer exists and an electron emerges from the atom with kinetic (motion in relation to force) energy almost equal to the gamma energy. This effect is predominant at low gamma energies and is known as the photoelectric effect. The other major effect occurs when a gamma ray interacts with an atomic electron, freeing it from the atom and imparting to it only a fraction of the gamma ray's kinetic energy. A secondary gamma ray with less energy (hence lower frequency) also emerges from the interaction. This effect predominates at higher gamma energies and is known as the Compton effect.
In both of these effects the emergent electrons lose their kinetic energy by ionizing surrounding atoms. The density of ions so generated is a measure of the energy delivered to the material by the gamma rays.
The most common means of measuring the variations in a beam of radiation is by observing its effect on a photographic film. This effect is the same as that of light, and the more intense the radiation is, the more it darkens, or exposes, the film. Other methods are in use, such as the ionizing effect measured electronically, its ability to discharge an electrostatically charged plate or to cause certain chemicals to fluoresce as in fluoroscopy.
Obsolete terminology[edit | edit source]
The term skiagrapher was used until about 1918 to mean radiographer. It was derived from Ancient Greek words for 'shadow' and 'writer'.
See also[edit | edit source]
- CAD Systems (Computer Aided Diagnosis)
- Radiation
- Radiation contamination
- List of civilian radiation accidents
- Radiographer
- Projectional radiography
References[edit | edit source]
- Kodak. (http://www.kodak.com/global/en/health/productsByType/index.jhtml?pq-path=2/521/2970)
- Agfa. (http://www.piribo.com/publications/medical_devices/mdc/agfa_medical.html)
- A review on the subject of medical X-ray examinations and metal based contrast agents, by Shi-Bao Yu and Alan D. Watson, Chemical Reviews, 1999, volume 99, pages 2353-2378
- Composite Materials for Aircraft Structures by Alan Baker, Stuart Dutton (Ed.), AIAA (American Institute of Aeronautics & Ast) ISBN 1-56347-540-5
External links[edit | edit source]
- NIST's XAAMDI: X-Ray Attenuation and Absorption for Materials of Dosimetric Interest Database
- NIST's XCOM: Photon Cross Sections Database
- NIST's FAST: Attenuation and Scattering Tables
- American College of Radiology
- Major John Hall-Edwards, British radiography pioneer
- A lost industrial radiography source event
- UN information on the security of industrial sources
- RadiologyInfo - The radiology information resource for patients: Radiography (X-rays)
- The Society of Radiographers Definitive information on the practice of Radiography Professionals
- Sumer's Radiology Site Radiology Blog working as an Online Radiology Magazine
- Nick Oldnall's radiography site
- EUROPEAN SOCIETY OF RADIOLOGY
zh-min-nan:Tiān-kong liap-iáⁿ de:Röntgen eu:Erradiografia it:Radiografia convenzionale he:צילום רנטגן nl:Röntgenfoto nrm:X-raiethie uk:Радіографія
 KSF
KSF