Signal transduction
 From Wikidoc - Reading time: 21 min
From Wikidoc - Reading time: 21 min
Overview[edit | edit source]
In biology, signal transduction refers to any process by which a cell converts one kind of signal or stimulus into another, most often involving ordered sequences of biochemical reactions inside the cell, that are carried out by enzymes and linked through second messengers resulting in what is thought of as a "second messenger pathway". Such processes are usually rapid, lasting on the order of milliseconds in the case of ion flux, to minutes for the activation of protein and lipid mediated kinase cascades. In many signal transduction processes, the number of proteins and other molecules participating in these events increases as the process eminates from the initial stimulus, resulting in a "signal cascade" and often results in a relatively small stimulus eliciting a large response.
In bacteria and other single-cell organisms, the variety of signal transduction processes of which the cell is capable influences how many ways it can react and respond to its environment. In multicellular organisms, a multitude of different signal transduction processes are required for coordinating the behavior of individual cells to support the function of the organism as a whole. As may be expected, the more complex the organism, the more complex the repertoire of signal transduction processes the organism must possess. Thus, sensing of both the external and internal environment at the cellular level, relies on signal transduction. Many disease processes such as diabetes, heart disease, autoimmunity and cancer arise from defects in signal transduction pathways, further highlighting the critical importance of signal transduction to biology as well as medicine.
History[edit | edit source]

The earliest published scientific paper recorded in the MEDLINE database as containing the specific term "signal transduction" within its text was published in 1972.[1] Prior to 1977 articles can be found that use the term "signal transmission" or "sensory transduction" within their title or abstract.[2][3] However it is not until 1977 that papers start to appear with the specific term "signal transduction" within their abstract, and 1979 before this specific term appears within a paper title.[4][5] One source attributes the widespread use of the term signal transduction to a 1980 review article by Rodbell.[6][7]
As can be seen from the graph to the right it wasn't until the late 1980s/early 1990s that research papers directly addressing signal transduction processes began to appear in large numbers in the scientific literature. The occurrence of a specific term within the title or abstract of a scientific paper is usually a good indicator that the paper addresses a specifically related area of research. While there may be considered to be a number of landmark or important discoveries in the field of signal transduction, such as the link made by Rodbell between metabolic regulation and the activity of GTP and GTP-binding proteins,[7] much of our current understanding of signal transduction processes is as a result of numerous contributions made to the field over many years by numerous different research groups all over the world.
The total number of scientific papers related to signal transduction published since 1st Jan 1977 up to the 31st December 2007 was 48,377 of which only 11,211 were reviews of other papers
Signaling Molecules[edit | edit source]
Signal transduction usually involves the binding of small extracellular signaling molecules to receptors that face outwards from the plasma membrane and trigger events inside the cell. However, steroids represent an example of extracellular signalling molecules that may cross the plasma membrane due to their lipophilic or hydrophobic nature.[8] Many steroids, but not all, have receptors within the cytoplasm and usually act by stimulating the binding of their receptors to the promoter region of steroid responsive genes.[9] Within multicellular organisms there are a diverse number of small molecules and polypeptides that serve to coordinate a cell's individual biological activity within the context of the organism as a whole. These molecules have been functionally classified as:
- hormones (e.g. melatonin),[10]
- growth factors (e.g. epidermal growth factor),[11]
- extra-cellular matrix components (e.g. fibronectin),[12]
- cytokines (e.g. interferon-gamma),[13]
- chemokines (e.g. RANTES),[14]
- neurotransmitters (e.g. acetylcholine),[15] and
- neurotrophins (e.g. nerve growth factor).[16]
It is important to note that most of these classifications do not take into account the molecular nature of each class member. For example, as a class, neurotransmitters consist of neuropeptides such as endorphins[17] and small molecules such as serotonin[18] and dopamine.[19] Hormones are also a generic class of molecule able to initiate signal transduction, these include insulin (a polypeptide),[20] testosterone (a steroid),[21] and epinephrine (an amino acid derivative, in essence a small organic molecule).[22]
The classification of one molecule into one class of another is not exact. For example, epinephrine and norepinephrine secreted by the central nervous system act as neurotransmitters. However, epinephrine when secreted by the adrenal medulla acts as a hormone.
Environmental stimuli[edit | edit source]
In addition to many of the regular signal transduction stimuli listed above, in complex organisms, there are also examples of additional environmental stimuli that initiate signal transduction processes. Environmental stimuli may also be molecular in nature (as above) or more physical, such as, light striking cells in the retina of the eye,[23] odorants binding to odorant receptors in the nasal epithelium,[24] and bitter and sweet tastes stimulating taste receptors in the taste buds.[25] Certain microbial molecules e.g. viral nucleotides, bacterial lipopolysaccharides, or protein antigens are able to elicit an immune system response against invading pathogens, mediated via signal transduction processes. An immune response may occur independently from signal transduction stimulation by other molecules, as is the case for signal transduction via the Toll-like receptor or with help from stimulatory molecules located at the cell surface of other cells, as is the case for T-cell receptor signalling.
Unicellular organisms may also respond to environmental stimuli via the activation of signal transduction pathways. For example slime molds secrete cyclic AMP upon starvation which stimulates individual cells in the immediate environment to aggregate.[26] Yeast also use mating factors to determine the mating types of other yeast and participate in sexual reproduction.[27]
If a cell lacks the specific receptor protein for detection of a particular stimulus, then the cell can be said to be "blind" to the stimulus.
Cellular responses[edit | edit source]
Activation of genes,[28] alterations in metabolism,[29] the continued proliferation and death of the cell,[30] and the stimulation or suppression of locomotion,[31] are some of the cellular responses to extracellular stimulation that require signal transduction. Gene activation leads to further cellular effects, since the protein products of many of the responding genes include enzymes and transcription factors themselves. Transcription factors produced as a result of a signal transduction cascade can in turn activate yet more genes. Therefore an initial stimulus can trigger the expression of an entire cohort of genes, and this in turn can lead to the activation of any number of complex physiological events. These events include the increased uptake of glucose from the blood stream stimulated by insulin[29] and the migration of neutrophils to sites of infection stimulated by bacterial products. The set of genes and the order in which they are activated in response to stimuli are often referred to as a "genetic program".[32]
Neurotransmitters are ligands that are capable of binding to ion channel proteins resulting in their opening to allow the rapid flow of a particular ion across the plasma membrane.[15] This results in an altering of the cell's membrane potential and is important for processes such as the neural conduction of electrochemical impulses. Ligands can be freely soluble,[11] or can be found on the surface of other cells or within the extracellular matrix.[12] Such cell surface or extracellular matrix ligands signal between cells when they come in contact with each other, such as when a phagocytic cell presents antigens to lymphocytes, or upon adhesion to the extracellular matrix, as when integrins at the cell surface of fibroblasts engage fibronectin.[33]
Most mammalian cells require stimulation to control not only cell division, but also survival. In the absence of growth factor stimulation, programmed cell death ensues in most cells. Such requirements for extra-cellular stimulation are necessary for controlling cell behavior in both the context of unicellular and multi-cellular organisms. Signal transduction pathways are so central to biological processes that it is not surprising that a large number of diseases have been attributed to their dysregulation.
Discussed below are how signal transduction via various classes of receptor may lead to the above cellular responses.
Types of receptor[edit | edit source]
Receptors can be roughly divided into two major classes:
- Intracellular receptors and
- Cell-surface receptors.
Ligand gated ion channel receptors are a class of receptor that may occur both at the cell-surface or intracellularly.
Receptors that are solely intracellular include those for steroid hormones, thyroid hormone, retinoic acid and derivatives of vitamin D3. In contrast to ligands that bind to cell surface receptors, in order to initiate signal transduction these ligands must cross the cell membrane. See the intracellular receptors section below for more details.
Cell-surface receptors[edit | edit source]
Cell-surface receptors are integral transmembrane proteins and recognise the vast majority of extracellular signaling molecules. Transmembrane receptors span that the plasma membrane of the cell, with one part of the receptor on the outside of the cell (the extracellular domain) and the other on the inside of the cell (the intracellular domain). Signal transduction occurs as a result of stimulatory molecule or ligand binding to their extracellular domain, the ligand itself does not pass through the plasma membrane prior to receptor binding.
Binding of a ligand to a cell-surface receptor stimulates a series of events inside the cell with different types of receptor stimulating different intracellular responses. Receptors typically only respond to the binding of a specific ligand. Upon binding the ligand initiates the transmission of a signal across the plasma membrane by inducing a change in the shape or conformation of the intracellular part of the receptor. Often, such changes in conformation result in either the activation of an enzymatic activity contained within the receptor or exposes a binding site for other signaling proteins within the cell. Once these proteins bind to the receptor they themselves may become active and propagate the signal into the cytoplasm.
In eukaryotic cells, most intracellular proteins activated by a ligand/receptor interaction possess an enzymatic activity. These enzymes include heterotrimeric G proteins, small GTPases, various protein kinases and phosphatases, lipid kinases and hydrolases. Some receptor stimulated enzymes create specific second messengers including cyclic nucleotides, such as cyclic AMP (cAMP) and cyclic GMP (cGMP), Phosphatidylinositol derivatives, such as Phosphatidylinositol-triphosphate (PIP3), Diacylglycerol (DAG) and Inositol-triphosphate (IP3). IP3 controlling the release of intracellular calcium stores into the cytoplasm (see second messengers section later in this article). Other activated proteins interact with adaptor proteins. Adaptor proteins facilitate interactions between other signaling proteins, and coordinate the formation of signaling complexes necessary in order to produce an appropriate cellular response to a particular stimulus. Enzymes and adaptor proteins are both responsive to various second messenger molecules.
Many of the enzymes activated as part of the signal transduction mechanism and also many adaptor proteins have been found to possess specialized protein domains that bind to specific secondary messenger molecules. For example, calcium ions bind specifically to the EF hand domains of calmodulin, allowing this molecule to bind and activate Calmodulin dependent kinase. PIP3 binds specifically to the Pleckstrin homology domains of proteins such as the kinase protein AKT again with an activatory activity.
There are many different classes of transmembrane receptor that recognise many different extracellular signalling molecules. Specific example receptors discussed in this article are:
- G-protein coupled receptors - e.g. Chemokine receptors
- Receptor tyrosine kinases - e.g. Growth factor receptors,
- Integrins
- Toll-like receptors
Further examples are given in the transmembrane receptor article.
G-protein coupled receptors[edit | edit source]
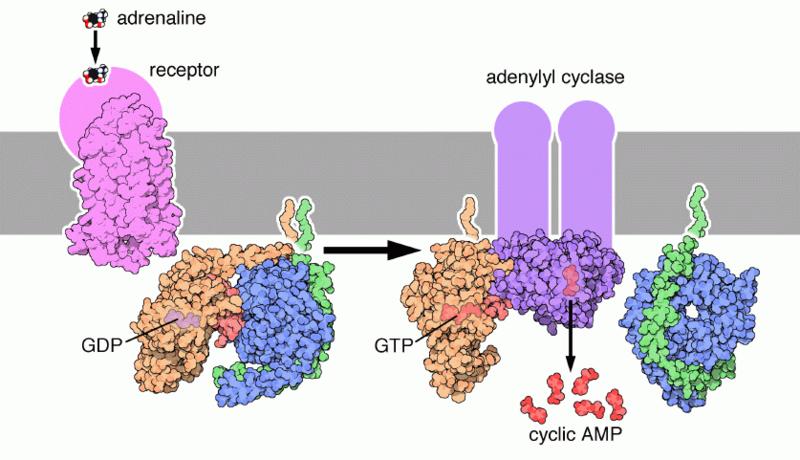
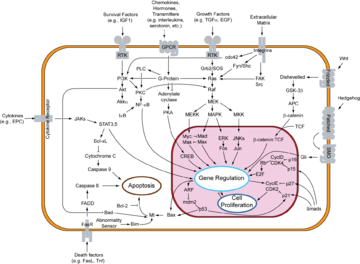
G-protein coupled receptors (GPCRs) are a family of integral membrane proteins that possess seven membrane-spanning domains, and are linked to a guanine nucleotide binding protein (or G protein). Many receptors make up this family, including adrenergic receptors, olfactory receptors, opioid receptors, chemokine receptors and rhodopsin.
Signal transduction by a GPCR put simply begins with an inactive G protein coupled with the receptor. An inactive G protein exists as a heterotrimer, a molecule composed of three different protein subunits. Once the GPCR recognizes a ligand, the shape (conformation) of the receptor changes to mechanically activate the G protein, and causes one subunit (Gα) to dissociate from the other two G-protein subunits (Gβ and Gγ); the dissociation exposes sites on the G-protein subunits that interact with other molecules.[34] The activated G protein subunits detach from the receptor and initiate signaling from many downstream effector proteins, including phosphodiesterases and adenylyl cyclases, phospholipases, and ion channels that permit the release of second messenger molecules such as cyclic AMP (cAMP), cyclic GMP (cGMP), inositol triphosphate (IP3), diacylglycerol (DAG), and calcium (Ca2+) ions.[35] For example, a rhodopsin molecule in the plasma membrane of a retina cell in the eye that was activated by a photon can activate up to 2000 effector molecules (in this case, transducin) per second.
The total strength of signal amplification by a GPCR is determined by:
- The lifetime of the ligand-receptor-complex. If the ligand-receptor-complex is stable, it takes longer for the ligand to dissociate from its receptor, thus the receptor will remain active for longer and will activate more effector proteins.
- The amount and lifetime of the receptor-effector protein-complex. The more effector protein is available to be activated by the receptor, and the faster the activated effector protein can dissociate from the receptor, the more effector protein will be activates in the same amount of time.
- Deactivation of the activated receptor. A receptor that is engaged in a hormone-receptor-complex can be deactivated, either by covalent modification (for example, phosphorylation), or by internalization (see ubiquitin).
The idea that G-protein coupled receptors, specifically chemokine receptors participate in cancer development is suggested by a study where a point mutation was inserted into the gene encoding the chemokine receptor CXCR2. Cells transfected with the CXCR2 mutant underwent a malignant transformation.[36] The result of the point mutation was the expression of CXCR2 in an active conformation, despite the absence of chemokine binding (the CXCR2 mutant is said to be "constitutively active").
Receptor Tyrosine Kinases[edit | edit source]
Receptor tyrosine kinases (RTKs) are transmembrane proteins with an intracellular kinase domain and an extracellular domain that binds ligand. There are many RTK proteins that are classified into subfamilies depending on their structural properties and ligand specificity. These include many growth factor receptors such as insulin receptor and the insulin-like growth factor receptors, and many others receptors.[37] To conduct their biochemical signals, RTKs need to form dimers in the plasma membrane. The dimer is stabilized by ligand binding by the receptor. Interaction between the two cytoplasmic domains of the dimer is thought to stimulate autophosphorylation of tyrosines within the cytoplasmic tyrosine kinase domains of the RTKs causing their conformational changes. The kinase domain of the receptors is subsequently activated, initiating signaling cascades of phosphorylation of downstream cytoplasmic molecules. These signals are essential to various cellular processes, such as control of cell growth, differentiation, metabolism, and migration.[37]
As is the case with G-Protein coupled receptors, proteins that bind GTP play a major role in transmission of signal from the activated RTK into the cell. In this case the G proteins are members of the Ras, Rho and Ral families, referred to collectively as small G proteins. These proteins act as molecular switches that are usually tethered to membranes by isoprenyl groups linked to their carboxyl ends. Thus, upon activation they are responsible for the recruitment of proteins to specific membrane subdomains where they participate in signaling. Activated RTKs in turn activate small G proteins which in turn activate Guanine Nucleotide Exchange Factors, such as SOS1. Once activated, these exchange factors can activate many more small G-proteins, thus amplifying the receptors initial signal.
As with the mutation of G-protein coupled receptors, the mutation of certain RTK genes can result in the expression of receptors that exist in a constitutively activate state. Such mutated RTK genes may act as oncogenes, genes that contribute to the initiation or progression of cancer.[38]
Integrins[edit | edit source]

Integrins are produced by a wide variety of cell types and play a role in the attachment of a cell to the extracellular matrix (ECM) and to other cells, and in the signal transduction of signals received from extracellular matrix components such as fibronectin, collagen and laminin. Ligand binding to the extracellular domain of integrins induce a conformational change within the protein and a clustering of the protein at the cell surface, in order to initiate signal transduction. Integrins lack kinase activity and integrin mediated signal transduction is achieved through a variety of intracellular protein kinases and adaptor molecules such as integrin-linked kinase (ILK), focal-adhesion kinase (FAK), talin, paxillin, parvins, p130Cas, Src-family kinases and GTPases of the Rho family. The main protein coordinating signal transduction being ILK.[39] As shown in the overview to the right, cooperative integrin and receptor tyrosine kinase signalling determins cellular survival, apoptosis, proliferation and differentiation.
Important differences exist between integrin signaling in circulating blood cells and non-circulating blood cells such as epithelial cells. Integrins at the cell-surface of circulating cells are inactive under normal physiological conditions. For example cell-surface integrins on circulating leukocytes are maintained in an inactive state in order to avoid epithelial cell attachment. Only in response to appropriate stimuli are leukocyte integrins converted into an active form, such as those received at the site of an inflamatory response. Similarly, it is important that integrins at the cell surface of circulating platelets are kept in an inactive state under normal conditions, in order to avoid thrombosis. Epithelial cells, in contrast have active integrins at their cell surface under normal conditions, which help to maintain their stable adhesion to underlying stromal cells, which provide appropriate signals in order to maintain their survival and differentiation.[40]
Toll-Like Receptors[edit | edit source]
When activated, Toll-like receptors (TLRs) recruit adapter molecules within the cytoplasm of cells in order to propagate a signal. Four adapter molecules are known to be involved in signaling. These proteins are known as MyD88, Tirap (also called Mal), Trif, and Tram.[41][42][43] The adapters activate other molecules within the cell, including certain protein kinases (IRAK1, IRAK4, TBK1, and IKKi) that amplify the signal, and ultimately lead to the induction or suppression of genes that orchestrate the inflammatory response. In all, thousands of genes are activated by TLR signaling, and collectively, the TLRs constitutes one of the most powerful and important gateways for gene modulation.
Ligand-gated ion channel receptors[edit | edit source]
A ligand-activated ion channel will recognize its ligand, and then undergo a structural change that opens a gap (channel) in the plasma membrane through which ions can pass. These ions will then relay the signal. An example for this mechanism is found in the receiving cell, or post-synaptic cell of a neural synapse.
By contrast, other ion channels open in response to a change in cell potential, that is, the difference of the electrical charge across the membrane. In neurons, this mechanism underlies the action potentials that travel along nerves. The influx of ions that occurs in response to ligand gated ion channels often induce action potentials by depolarizing the membrane of the post-synaptic cells which results in the wave like opening of voltage gated ion channels. In addition, calcium ions are also commonly allowed into the cell during ligand induced ion channel opening. This calcium can act as a classical second messenger, setting in motion signal transduction cascades and altering the cellular physiology of the responding cell. This may result in strengthening of the synapse between the pre- and post synaptic cells by remodeling the dendritic spines involved in the synapse.
Intracellular receptors[edit | edit source]
Intracellular receptors include nuclear receptors and cytoplasmic receptors, and are soluble proteins localized within the nucleoplasm or the cytoplasm respectively. The typical ligands for nuclear receptors are lipophilic hormones, with steroid hormones (for example, testosterone, progesterone and cortisol) and derivatives of vitamin A and D among them. In order to reach its receptor and initiate signal transduction, the hormone must pass through the plasma membrane, usually by passive diffusion. The nuclear receptors are ligand-activated transcription activators; on binding with the ligand (the hormone), they will pass through the nuclear membrane into the nucleus and enable the transcription of a certain gene and, thus, the production of a protein.
The nuclear receptors that were activated by the hormones attach at the DNA at receptor-specific Hormone Responsive Elements (HREs), DNA sequences that are located in the promoter region of the genes that are activated by the hormone-receptor complex. As this enables the transcription of the according gene, these hormones are also called inductors of gene expression. The activation of gene transcription is much slower than signals that directly affect existing proteins. As a consequence, the effects of hormones that use nucleic receptors are usually long-term. Although the signal transduction via these soluble receptors involves only a few proteins, the details of gene regulation are yet not well understood. The nucleic receptors all have a similar, modular structure:
- N-AAAABBBBCCCCDDDDEEEEFFFF-C
where CCCC is the DNA-binding domain that contains zinc fingers, and EEEE the ligand-binding domain. The latter is also responsible for dimerization of most nuclearic receptors prior to DNA binding. As a third function, it contains structural elements that are responsible for transactivation, used for communication with the translational apparatus. The zinc fingers in the DNA-binding domain stabilize DNA binding by holding contact to the phosphate backbone of the DNA. The DNA sequences that match the receptor are usually hexameric repeats, either normal, inverted or everted. The sequences are quite similar, but their orientation and distance are the parameters by which the DNA-binding domains of the receptors can tell them apart.
Steroid receptors are a subclass of nuclear receptors, located primarily within the cytosol. In the absence of steroid hormone, the receptors cling together in a complex called an aporeceptor complex, which also contains chaperone proteins (also known as heatshock proteins or Hsps). The Hsps are necessary to activate the receptor by assisting the protein to fold in a way such that the signal sequence which enables its passage into the nucleus is accessible.
Steroid receptors can also have a repressive effect on gene expression, when their transactivation domain is hidden so it cannot activate transcription. Furthermore, steroid receptor activity can be enhanced by phosphorylation of serine residues at their N-terminal end, as a result of another signal transduction pathway, for example, a by a growth factor. This behaviour is called crosstalk.
RXR- and orphan-receptors These nuclear receptors can be activated by
- a classic endocrine-synthesized hormone that entered the cell by diffusion.
- a hormone that was built within the cell (for example, retinol) from a precursor or prohormone, which can be brought to the cell through the bloodstream.
- a hormone that was completely synthesized within the cell, for example, prostaglandin.
These receptors are located in the nucleus and are not accompanied by chaperone proteins. In the absence of hormone, they bind to their specific DNA sequence, repressing the gene. Upon activation by the hormone, they activate the transcription of the gene they were repressing.
Certain intracellular receptors of the immune system are examples of cytoplasmic receptors. Recently identified NOD like receptors (NLRs) reside in the cytoplasm of specific eukaryotic cells and interact with particular ligands, such as microbial molecules, using a leucine-rich repeat (LRR) motif that is similar to the ligand binding motif of the extracellular receptors known as TLRs. Some of these molecules (e.g. NOD1 and NOD2) interact with an enzyme called RICK kinase (or RIP2 kinase) that activates NF-κB signaling, while others (e.g. NALP3) interact with inflammatory caspases (e.g. caspase 1) and initiate processing of particular cytokines (e.g. interleukin-1β).[44] Similar receptors exist inside plant cells and are called Plant R Proteins. Another type of cytoplasmic receptor also has a role in immune surveillance. These receptors are known as RNA Helicases and include RIG-I, MDA5 and LGP2.[45]
Second Messengers[edit | edit source]
Intracellular signal transduction is largely carried out by second messenger molecules.
Ca2+ concentration is usually maintained at a very low level in the cytosol by sequestration in the smooth endoplasmic reticulum and the mitochondria. Ca2+ release from the endoplasmic reticulum into the cytosol results in the binding of the released Ca2+ to signaling proteins that are then activated. There are two combined receptor/ion channel proteins that perform the task of controlled transport of Ca2+:
- The InsP3-receptor will transport Ca2+ upon interaction with inositol triphosphate (thus the name) on its cytosolic side. It consists of four identical subunits.
- The ryanodine receptor is named after the plant alkaloid ryanodine. It is similar to the InsP3 receptor and stimulated to transport Ca2+ into the cytosol by recognizing Ca2+ on its cytosolic side, thus establishing a feedback mechanism; a small amount of Ca2+ in the cytosol near the receptor will cause it to release even more Ca2+. It is especially important in neurons and muscle cells. In heart and pancreas cells, another second messenger (cyclic ADP ribose) takes part in the receptor activation. The localized and time-limited activity of Ca2+ in the cytosol is also called a Ca2+ wave. Once released into the cytosol from intracellular stores or extracellular sources, Ca2+ acts as a signal molecule within the cell. This works by tightly limiting the time and space when Ca2+ is free (and thus active). Therefore, the concentration of free Ca2+ within the cell is usually very low; it is stored within organelles, usually the endoplasmic reticulum (sarcoplasmic reticulum in muscle cells), where it is bound to molecules like calreticulin.
Ca2+ is used in a multitude of processes, among them muscle contraction, release of neurotransmitter from nerve endings, vision in retina cells, proliferation, secretion, cytoskeleton management, cell migration, gene expression and metabolism. The three main pathways that lead to Ca2+ activation are :
- G protein regulated pathways
- Pathways regulated by receptor-tyrosine kinases
- Ligand- or current-regulated ion channels
There are two different ways in which Ca2+ can regulate proteins:
- A direct recognition of Ca2+ by the protein.
- Binding of Ca2+ in the active site of an enzyme
One of the best studied interactions of Ca2+ with a protein is the regulation of calmodulin by Ca2+. Calmodulin itself can regulate other proteins, or be part of a larger protein (for example, phosphorylase kinase). The Ca2+/calmodulin complex plays an important role in proliferation, mitosis and neural signal transduction.
Lipophilic second messenger molecules These molecules are all derived from lipids that normally reside in cellular membranes. Enzymes stimulated by activated receptors modify these lipids, converting them into second messengers. One example of lipophilic second messenger molecule is diacylglycerol, required for the activation of protein kinase C. Others are ceramide, the eicosanoids and lysophosphatidic acid.
Nitric oxide (NO) as second messenger The gas nitric oxide is a free radical which diffuses through the plasma membrane and affects nearby cells. NO is made from arginine and oxygen by the enzyme NO synthase, with citrulline as a by-product. NO works mainly through activation of its target receptor, the enzyme soluble guanylate cyclase, which when activated, produces the second messenger cyclic guanosine monophosphate (cGMP). NO can also act through covalent modification of proteins or their metal cofactors. Some of these modifications are reversible and work through a redox mechanism. In high concentrations, NO is toxic, and is thought to be responsible for some damage after a stroke. NO serves multiple functions. These include:
- Relaxation of blood vessels.
- Regulation of exocytosis of neurotransmitters.
- Cellular immune response.
- Modulation of the Hair Cycle.
- Production and maintenance of penile erections.
- Activation of apoptosis by initiating signals which lead to H2AX phosphorylation
See also[edit | edit source]
- Functional selectivity
- G protein-coupled receptor -- GTPases -- Protein phosphatase
- MAPK/ERK pathway - a signal transduction pathway linking cell surface receptors to transcription factors.
- Redox signaling
References[edit | edit source]
- ↑ Rensing, L. (1972). "Periodic geophysical and biological signals as Zeitgeber and exogenous inducers in animal organisms". Int. J. Biometeorol. 16: Suppl:113-125. PMID 4621276.
- ↑ Tonndorf J. (1975). "Davis-1961 revisited. Signal transmission in the cochlear hair cell-nerve junction". Arch. Otolaryngol. 101 (9): 528–535. PMID 169771.
- ↑ Ashcroft SJ, Crossley JR, Crossley PC. (1976). "The effect of N-acylglucosamines on the biosynthesis and secretion of insulin in the rat". Biochem. J. 154 (3): 701–707. PMID 782447.
- ↑ Hildebrand E. (1977). "What does Halobacterium tell us about photoreception?". Biophys. Struct. Mech. 3 (1): 69–77. PMID 857951.
- ↑ Kenny JJ, Martinez-Maza O.; et al. (1979). "Lipid synthesis: an indicator of antigen-induced signal transduction in antigen-binding cells". J. Immunol. 112 (4): 1278–1284. PMID 376714.
- ↑ Gomperts, BD. (2002). Signal transduction. Academic Press. ISBN 0-12-289631-9. Unknown parameter
|coauthors=ignored (help) - ↑ 7.0 7.1 Rodbell, M. (1980). "The role of hormone receptors and GTP-regulatory proteins in membrane transduction". Nature. 284 (5751): 17–22. PMID 6101906.
- ↑ Beato M, Chavez S and Truss M (1996). "Transcriptional regulation by steroid hormones". Steroids. 61 (4): 240–251. PMID 8733009.
- ↑ Hammes SR (2003). "The further redefining of steroid-mediated signaling". Proc Natl Acad Sci USA. 100 (5): 21680–2170. PMID 12606724.
- ↑ Sugden D, Davidson K.; et al. (2004). "Melatonin, melatonin receptors and melanophores: a moving story". Pigment Cell Res. 17 (5): 454–460. PMID 15357831.
- ↑ 11.0 11.1 Carpenter G, and Cohen S. (1990). "Epidermal growth factor". J. Biol. Chem. 265 (14): 7709–7712. PMID 2186024.
- ↑ 12.0 12.1 Ward M, and Marcey, D. [1] Retrieved on 2007-03-06
- ↑ Schroder; et al. (2004). "Interferon-γ an overview of signals, mechanisms and functions". Journal of Leukocyte Biology. 75: 163–189.
- ↑ Chung CW, Cooke RM; et al. (1995). "The three-dimensional solution structure of RANTES". Biochemistry. 34 (29): 9307–9314. PMID 7542919.
- ↑ 15.0 15.1 Kistler J, Stroud RM; et al. (1982). "Structure and function of an acetylcholine receptor". Biophys. J. 37 (1): 371–383. PMID 7055628.
- ↑ Wiesmann, C. and de Vos, AM. (2001). "Nerve growth factor: structure and function". Cell Mol Life Sci. 58 (5–6): 748–759. PMID 11437236.
- ↑ Goldstein, A. (1976). "Opioid peptides endorphins in pituitary and brain". Science. 193 (4258): 1081–1086. PMID 959823.
- ↑ Kroeze WK, Kristiansen K, and Roth BL. (2002). "Molecular biology of serotonin receptors, structure and function at the molecular level". Curr Top Med Chem. 2 (6): 507–528. PMID 12052191.
- ↑ Missale C, Nash SR.; et al. (1998). "Dopamine receptors:from structure to function". Physiol. Rev. 78 (1): 189–225. PMID 9457173.
- ↑ Adams TE, Epa, VC; et al. (2000). "Structure and function of the type 1 insulin-like growth factor receptor". Cell Mol Life Sci. 57 (7): 1050–1093. PMID 10961344.
- ↑ Roy AK and Chatterjee B. (1995). "Androgen action". Crit Rev Eukaryot Gene Expr. 5 (2): 157–176. PMID 8845582.
- ↑ Small KM, McGraw DW and Liggett SB. (2003). "Pharmacology and physiology of human adrenergic receptor polymorphisms". Annu Rev Pharmacol Toxicol. 43: 381–411. PMID 12540746.
- ↑ Burns ME and Arshavsky VY. (2005). "Beyond counting photons: trials and trends in vertebrate visual transduction". Neuron. 48 (3): 387–401. PMID 16269358.
- ↑ Ronnett GV and Moon C. (2002). "G proteins and olfactory signal transduction". Annu Rev Physiol. 64: 189–222. PMID 11826268.
- ↑ Wong GT, Gannon KS and Margolskee RF. (1996). "Transduction of bitter and sweet taste by gustducin". Nature. 381 (6585): 796–800. PMID 8657284.
- ↑ Hanna MH, Nowicki JJ and Fatone MA (1984). "Extracellular cyclic AMP during development of the cellular slime mold Polysphondylium violaceum: comparison of accumulation in the wild type and an aggregation-defective mutant". J Bacteriol. 157 (2): 345–349. PMID 215252.
- ↑ Sprague GF Jr. (1991). "Signal transduction in yeast mating: receptors, transcription factors, and the kinase connection". Trends Genet. 7 (11–12): 393–398. PMID 1668192.
- ↑ Lalli E and Sassone-Corsi P (1994). "Signal transduction and gene regulation: the nuclear response to cAMP". j Biol Chem. 269 (26): 17359–17362. PMID 8021233.
- ↑ 29.0 29.1 Rosen O (1987). "After insulin binds". Science. 237 (4821): 1452–1458. PMID 2442814.
- ↑ Guo D, Jia Q.; et al. (1995). "Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation". J Biol Chem. 270 (12): 6729–6733. PMID 7896817.
- ↑ Bornfeldt KE, Raines EW.; et al. (1995). "Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation". Ann N Y Acad Sci. 766: 416–430. PMID 7486687.
- ↑ Massague J and Gomis RR (2006). "The logic of TGFbeta signaling". FEBS Lett. 580 (12): 2811–2820. PMID 16678165.
- ↑ Johansson S. Svineng G; et al. (1997). "Fibronectin-integrin interactions". Front. Biosci. 2: d126–146. PMID 9159220.
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer; Web content by Neil D. Clarke (2002). Biochemistry. San Francisco: W.H. Freeman. ISBN 0-7167-4954-8.
- ↑ Yang W, Xia S (2006). "Mechanisms of regulation and function of G-protein-coupled receptor kinases". World J Gastroenterol. 12 (48): 7753–7. PMID 17203515.
- ↑ Burger M, Burger, JA; et al. (1999). "Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi's sarcoma herpesvirus-G protein-coupled receptor". J. Immunol. 163 (4): 2017–2022. PMID 10438939.
- ↑ 37.0 37.1 Li E, Hristova K (2006). "Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies". Biochemistry. 45 (20): 6241–51. PMID 16700535.
- ↑ Roskoski, R, Jr. (2004). "The ErbB/HER receptor protein-tyrosine kinases and cancer". Biochem. Biophys. Res. Commun. 319 (1): 1–11. PMID 15158434.
- ↑ 39.0 39.1 Hehlgans, S. Haase, M. and Cordes, N. (2007). "Signaling via integrins: Implications for cell survival and anticancer strategies". Biochim. Biophys. Acta. 1775 (1): 163–180. PMID 17084981.
- ↑ Gilcrease MZ. (2006). "Integrin signaling in epithelial cells". Cancer Lett. 247 (1): 1–25. PMID 16725254.
- ↑ Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003). "Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway". Science. 301 (5633): 640–3. PMID 12855817.
- ↑ Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S (2003). "TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway". Nat Immunol. 4 (11): 1144–50. PMID 14556004.
- ↑ Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S (2002). "Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4". Nature. 420 (6913): 324–9. PMID 12447441.
- ↑ Delbridge L, O'Riordan M (2007). "Innate recognition of intracellular bacteria". Curr Opin Immunol. 19 (1): 10–6. PMID 17126540.
- ↑ Fujita T, Onoguchi K, Onomoto K, Hirai R, Yoneyama M. "Triggering antiviral response by RIG-I-related RNA helicases". Biochimie. PMID 17379377.
Further reading[edit | edit source]
- Non-technical
- Cosma Shalizi's "Signal transduction" Notebook from 2003-01-20 used under the GFDL with permission
- Werner R. Loewenstein, The Touchstone of Life: Molecular Information, Cell Communication, and the Foundations of Life, Oxford University Press, 1999, ISBN 0-19-514057-5
- Technical
- Gomperts, Kramer, Tatham, "Signal Transduction", AP/Elsevier [2002], ISBN 0122896319. Reference book, for more information: http://www.cellbiol.net .
- Gerhard Krauss, Biochemistry of Signal Transduction and Regulation, Wiley-VCH, 1999, ISBN 3-527-30378-2
- John T. Hancock, Cell Signalling, Addison-Wesley, 1998 ISBN 0-582-31267-1
External links[edit | edit source]
- Signal Transduction - The Virtual Library of Biochemistry and Cell Biology
- TRANSPATH(R) - A database about signal transduction pathways
- Science's STKE - Signal Transduction Knowledge Environment, from the journal Science, published by AAAS.
- Signal+Transduction at the US National Library of Medicine Medical Subject Headings (MeSH)
- UCSD-Nature Signaling Gateway, from Nature Publishing Group
ar:توصيل الإشارة da:Signaltransduktion de:Signaltransduktion it:Trasduzione del segnale nl:Signaaltransductie simple:Signal transduction Template:Jb1
 KSF
KSF