Silver
 From Wikidoc - Reading time: 14 min
From Wikidoc - Reading time: 14 min
Template:Elementbox header Template:Elementbox series Template:Elementbox groupperiodblock Template:Elementbox appearance img Template:Elementbox atomicmass gpm Template:Elementbox econfig Template:Elementbox epershell Template:Elementbox section physicalprop Template:Elementbox color Template:Elementbox phase Template:Elementbox density gpcm3nrt Template:Elementbox densityliq gpcm3mp Template:Elementbox meltingpoint Template:Elementbox boilingpoint Template:Elementbox heatfusion kjpmol Template:Elementbox heatvaporiz kjpmol Template:Elementbox heatcapacity jpmolkat25 Template:Elementbox vaporpressure katpa Template:Elementbox section atomicprop Template:Elementbox crystalstruct Template:Elementbox oxistates Template:Elementbox electroneg pauling Template:Elementbox ionizationenergies3 Template:Elementbox atomicradius pm Template:Elementbox atomicradiuscalc pm Template:Elementbox covalentradius pm Template:Elementbox vanderwaalsrad pm Template:Elementbox section miscellaneous Template:Elementbox magnetic Template:Elementbox eresist ohmmat20 Template:Elementbox thermalcond wpmkat300k Template:Elementbox thermaldiff wpmkat300k Template:Elementbox thermalexpansion umpmkat25 Template:Elementbox speedofsound rodmpsatrt Template:Elementbox youngsmodulus gpa Template:Elementbox shearmodulus gpa Template:Elementbox bulkmodulus gpa Template:Elementbox poissonratio Template:Elementbox mohshardness Template:Elementbox vickershardness mpa Template:Elementbox brinellhardness mpa Template:Elementbox cas number |- ! colspan="2" style="background:#ffc0c0; color:black" | Selected isotopes |- | colspan="2" |
| iso | NA | half-life | DM | DE (MeV) | DP
Template:Elementbox isotopes decay2 Template:Elementbox isotopes decay2 Template:Elementbox isotopes stable Template:Elementbox isotopes decay3 Template:Elementbox isotopes stable Template:Elementbox isotopes decay2 Template:Elementbox isotopes end Template:Elementbox footer
Overview[edit | edit source]Silver (Template:PronEng) is a chemical element with the symbol "Ag" (Template:Lang-la) and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal. It occurs as a free metal (native silver) as well as in various minerals, such as argentite and chlorargyrite. Most silver is produced as a by-product of copper, gold, lead, and zinc mining. Silver has been known since antiquity, and it is used as a currency metal. It has long been valued as a precious metal used in ornaments and jewellery and in high-value tableware and utensils (hence the term "silverware"). Today, it is used in photographic film, electrical contacts and conductors, and mirrors. Elemental silver is also used to catalyze chemical reactions. Silver is antimicrobial, and dilute solutions of silver nitrate and other silver compounds are used as disinfectants. Although silver has largely been supplanted by other treatments, the antiseptic properties of silver are still a useful tool in the prevention and treatment of sepsis and infections caused by antibiotic-resistant microorganisms such as MRSA. Occurrence and extraction[edit | edit source]Silver is found in native form; combined with sulfur, arsenic, antimony, or chlorine; and in various ores, such as argentite (Ag2S), horn silver (AgCl), and pyrargyrite (Ag3SbS3). The principal sources of silver are the ores of copper, copper-nickel, gold, lead, and lead-zinc obtained from Peru, Mexico, China, and Australia. Peru and Mexico have been mining silver since 1546 and are still major world producers. The metal can also be produced during the electrolytic refining of copper and by application of the Parkes process on lead metal obtained from lead ores that contain small amounts of silver. Commercial-grade fine silver is at least 99.9% pure silver, and purities greater than 99.999% are available. In 2005, Peru was the top producer of silver with almost one-seventh world share, closely followed by Mexico, according to the British Geological Survey. Notable characteristics[edit | edit source] Silver is a very ductile and malleable (slightly harder than gold) monovalent coinage metal with a brilliant white metallic luster that can take a high degree of polish. It has the highest electrical conductivity of all metals, even higher than copper, but its greater cost and tarnishability have prevented it from being widely used in place of copper for electrical purposes, though it was used in the electromagnets used for enriching uranium during World War II (mainly because of the wartime shortage of copper). Another notable exception is in high-end audio cables, although the actual benefits of its use in this application are questionable. Among metals, pure silver has the highest thermal conductivity (only the non-metal diamond's is higher), whitest color, the highest optical reflectivity (although aluminium slightly outdoes it in parts of the visible spectrum, and it is a poor reflector of ultraviolet light). Silver also has the lowest contact resistance of any metal. Silver halides are photosensitive and are remarkable for their ability to record a latent image that can later be developed chemically. Silver is stable in pure air and water, but tarnishes when it is exposed to air or water containing ozone or hydrogen sulfide. The most common oxidation state of silver is +1 (for example, silver nitrate: AgNO3); a few +2 (for example, silver(II) fluoride: AgF2) and +3 compounds (for example, potassium tetrafluoroargentate: K[AgF4]) are also known. Isotopes[edit | edit source]Naturally occurring silver is composed of the two stable isotopes, 107Ag and 109Ag, with 107Ag being the more abundant (51.839% natural abundance). Silver's standard atomic mass is 107.8682(2) u. Twenty-eight radioisotopes have been characterised, the most stable being 105Ag with a half-life of 41.29 days, 111Ag with a half-life of 7.45 days, and 112Ag with a half-life of 3.13 hours. All of the remaining radioactive isotopes have half-lives that are less than an hour, and the majority of these have half-lives that are less than 3 minutes. This element has numerous meta states, the most stable being 108mAg (t* 418 years), 110mAg (t* 249.79 days) and 106mAg (t* 8.28 days). Isotopes of silver range in atomic weight from 93.943 u (94Ag) to 123.929 u (124Ag). The primary decay mode before the most abundant stable isotope, 107Ag, is electron capture and the primary mode after is beta decay. The primary decay products before 107Ag are palladium (element 46) isotopes, and the primary products after are cadmium (element 48) isotopes. The palladium isotope 107Pd decays by beta emission to 107Ag with a half-life of 6.5 million years. Iron meteorites are the only objects with a high-enough palladium-to-silver ratio to yield measurable variations in 107Ag abundance. Radiogenic 107Ag was first discovered in the Santa Clara meteorite in 1978. The discoverers suggest that the coalescence and differentiation of iron-cored small planets may have occurred 10 million years after a nucleosynthetic event. 107Pd–107Ag correlations observed in bodies that have clearly been melted since the accretion of the solar system must reflect the presence of live short-lived nuclides in the early solar system. Silver compounds[edit | edit source]
Applications[edit | edit source]As a precious metal[edit | edit source]A major use of silver is as a precious metal and it has long been used for making high-value objects reflecting the wealth and status of the owner. Jewellery and silverware are traditionally made from sterling silver (standard silver), an alloy of 92.5% silver with 7.5% copper. Sterling silver is harder than pure silver and has a lower melting point (893 °C) than either pure silver or pure copper. Britannia silver is an alternative hallmark-quality standard containing 95.8% silver, often used to make silver tableware and wrought plate. With the addition of germanium, the patented modified alloy Argentium Sterling Silver is formed, with improved properties including resistance to firescale. Silver is used in medals, denoting second place. Some high-end musical instruments are made from sterling silver, such as the flute. In dentistry[edit | edit source]The malleability and non-toxicity of silver make it useful in amalgams for dental fittings and fillings. In photography and electronics[edit | edit source]Photography used 24% of the silver consumed in 2001 in the form of silver nitrate and silver halides, while 33% was used in jewellery, 40% for industrial uses, and only 3% for coins and medals.[1] Some electrical and electronic products use silver for its superior conductivity, even when tarnished. For example, printed circuits are made using silver paints,[2] and computer keyboards use silver electrical contacts. Some high-end audio hardware (DACs, preamplifiers, etc.) are fully silver-wired, which is believed to cause the least loss of quality in the signal. Silver cadmium oxide is used in high voltage contacts because it can withstand arcing. 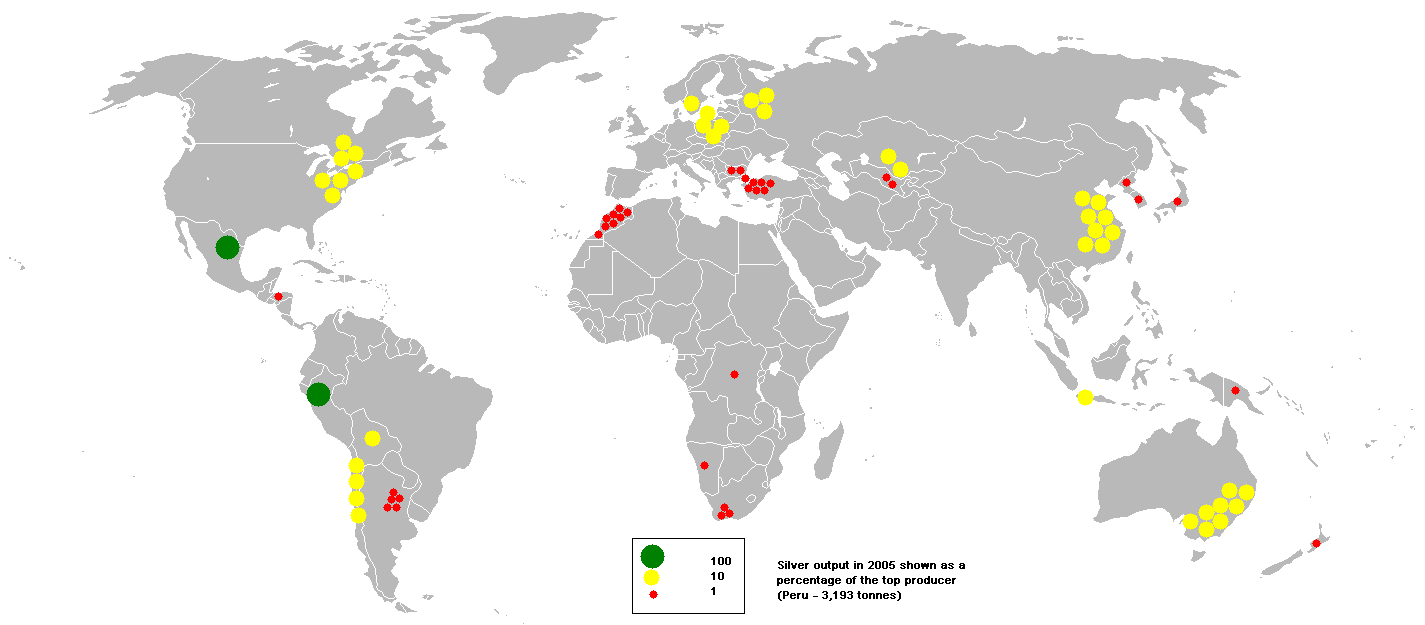   In solder and brazing[edit | edit source]Silver is also used to make solder and brazing alloys, electrical contacts, and high-capacity silver-zinc and silver-cadmium batteries. Silver in a thin layer on top of a bearing material can provide a significant increase in galling resistance and reduce wear under heavy load, particularly against steel. In mirrors and optics[edit | edit source]Mirrors which need superior reflectivity for visible light are made with silver as the reflecting material in a process called silvering, though common mirrors are backed with aluminium. Using a process called sputtering, silver (and sometimes gold) can be applied to glass at various thicknesses, allowing different amounts of light to penetrate. This is most often seen in architectural glass and tinted windows on vehicles. As a catalyst[edit | edit source]Silver's catalytic properties make it ideal for use as a catalyst in oxidation reactions, for example, the production of formaldehyde from methanol and air by means of silver screens or crystallites containing a minimum 99.95 weight-percent silver. Silver (upon some suitable support) is probably the only catalyst available today to convert ethylene to ethylene oxide (later hydrolyzed to ethylene glycol, used for making polyesters)—a very important industrial reaction. Oxygen dissolves in silver relatively easily compared to other gases present in air. Attempts have been made to construct silver membranes of only a few monolayers thickness. Such a membrane could be used to filter pure oxygen from air. As money[edit | edit source]Silver, in the form of electrum, was coined to produce money in around 700 BCE by the Lydians. Later, silver was refined and coined in its pure form (see silver coin). Many nations used silver as the basic unit of monetary value (see Silver standard). The words for "silver" and "money" are the same in at least 14 languages. In the modern world, silver bullion has the ISO currency code XAG. The name of the United Kingdom monetary unit "pound" reflects the fact that it originally represented the value of one troy pound of sterling silver. In the 1800s, many nations, such as the United States and Great Britain, switched from silver to a gold standard of monetary value, then in the 20th century to fiat currency. In medicine[edit | edit source]Silver ions and silver compounds show a toxic effect on some bacteria, viruses, algae and fungi, typical for heavy metals like lead or mercury, but without the high toxicity to humans that is normally associated with them. Its germicidal effects kill many microbial organisms in vitro. Hippocrates, the father of modern medicine, wrote that silver had beneficial healing and anti-disease properties, and the Phoenicians used to store water, wine, and vinegar in silver bottles to prevent spoiling. In the early 1900s people would put silver dollars in milk bottles to prolong the milk's freshness. Its germicidal effects increase its value in utensils and as jewellery. The exact process of silver's germicidal effect is still not well understood, although theories exist. One of these is the oligodynamic effect, which explains the effect on microbial lifeforms but does not explain certain antiviral effects. Silver compounds were used successfully to prevent infection in World War I before the advent of antibiotics. Silver nitrate solution was a standard of care but was largely replaced by silver sulfadiazine cream (SSD Cream)[3] which was generally the "standard of care" for the antibacterial and antibiotic treatment of serious burns until the late 1990s. Now, other options, such as silver-coated dressings (activated silver dressings), are used in addition to SSD cream and may present advantages such as pain reduction and capacity for treatment at home. The widespread use of silver went out of fashion with the development of modern antibiotics. However, recently there has been renewed interest in silver as a broad-spectrum antimicrobial. In particular, it is being used with alginate, a naturally occurring biopolymer derived from seaweed, in a range of silver alginate products designed to prevent infections as part of wound management procedures, particularly applicable to burn victims. In 2007, AGC Flat Glass Europe introduced the first antibacterial glass to fight hospital-caught infection: it is covered with a thin layer of silver.[4] In addition, Samsung has introduced washing machines with a final rinse containing silver ions to provide several days of antibacterial protection in the clothes.[5] Kohler has introduced a line of toilet seats that have silver ions embedded to kill germs. A company called Thomson Research Associates has begun treating products with Ultra Fresh, an anti-microbial technology involving "proprietary nano-technology to produce the ultra-fine silver particles essential to ease of application and long-term protection."[6] The FDA has recently approved an endotracheal breathing tube with a fine coat of silver for use in mechanical ventilation, after studies found it reduced the risk of ventilator-associated pneumonia.[7] As a medication[edit | edit source]Today, various kinds of silver compounds, or devices to make solutions or colloids containing silver, are sold as remedies for a wide variety of diseases. Although most are harmless, some people using these home-made solutions excessively have developed argyria over a period of months or years. Several cases have been documented in medical literature, including one case of coma associated with high intake of silver. It is strongly advised to consult a doctor before embarking on such treatment. Silver is widely used in topical gels and impregnated into bandages because of its wide-spectrum antimicrobial activity. The anti-microbial properties of silver stem from the chemical properties of its ionized form, Ag+. This ion forms strong molecular bonds with other substances used by bacteria to respire, such as molecules containing sulfur, nitrogen, and oxygen.[8] Once the Ag+ ion complexes with these molecules, they are rendered unusable by the bacteria, depriving it of necessary compounds and eventually leading to the bacteria's death. In food[edit | edit source]In India, foods, especially sweets, can be found decorated with a thin layer of silver known as vark. Silver as a food additive is given the E number E174 and is classed as a food coloring. It is used solely for external decoration, such as on chocolate confectionery, in the covering of dragées and the decoration of sugar-coated flour confectionery. In Australia, it is banned as a food additive. In clothing[edit | edit source]Silver inhibits the growth of bacteria and fungi. It keeps odour to a minimum and reduces the risk of bacterial and fungal infection. In clothing, the combination of silver and moisture movement (wicking) is the best combination to reduce the harmful effects of prolonged use in active and humid conditions. Silver is used in clothing in two main forms:
In both cases the silver prevents the growth of a broad spectrum of bacteria and fungi. At the same time, silver is a very skin-friendly and highly compatible agent to which – unlike many antibiotics – bacteria rarely build up resistance. Recorded use of silver to prevent infection dates to ancient Greece and Rome. It was rediscovered in the Middle Ages, where it was used for several purposes, such as to disinfect water and food during storage, and also for the treatment of burns and wounds as wound dressing. In the 19th century, sailors on long ocean voyages would put silver coins in barrels of water and wine to keep the liquid pure. Pioneers in America used the same idea as they made their journey from coast to coast. Silver solutions were approved in the 1920s by the US Food and Drug Administration for use as antibacterial agents. Today, wound dressings containing silver are well established for clinical wound care and have recently been introduced in consumer products such as sticking plasters.[citation needed] History[edit | edit source]The word "silver" appears in Anglo-Saxon in various spellings such as seolfor and siolfor. A similar form is seen throughout the Teutonic languages (compare Old High German silabar and silbir). The symbol "Ag" is from the Latin for "silver", argentum (compare Greek αργυρος (argyros)), from the Indo-European root arg- meaning "white" or "shining". Silver has been known since ancient times. It is mentioned in the book of Genesis, and slag heaps found in Asia Minor and on the islands of the Aegean Sea indicate that silver was being separated from lead as early as the 4th millennium BCE. Price[edit | edit source]Silver is currently about 1/50th the price of gold by mass and approximately 70 times more valuable than copper. Silver once traded at 1/6th to 1/12th the price of gold, prior to the Age of Discovery and the discovery of great silver deposits in the Americas, most notably the vast Comstock Lode in Virginia City, Nevada, USA. This then resulted in the debate over cheap Free Silver to benefit the agricultural sector, which was among the most prolonged and difficult in that country's history and dominated public discourse during the latter decades of the nineteenth century. Over the last 100 years the price of silver and the gold/silver price ratio have fluctuated greatly due to competing industrial and store-of-value demands. In 1980 the silver price rose to an all-time high of US$49.45 per troy ounce. By December 2001 the price had dropped to US$4.15 per ounce, and in May 2006 it had risen back as high as US$15.21 per ounce. As of 2006, silver prices (and most other metal prices) have been rather volatile, for example, quickly dropping from the May high of US$15.21 per ounce to a June low of US$9.60 per ounce before rising back above US$12.00 per ounce by August.[9] It is of note that in Judaic Law the price of silver is important. The lowest fiscal amount that a Jewish court, or Beth Din, can convene to adjudicate a case over is a shova pruta (value of a Babylonian prutra coin). This is fixed at 1/8 of a gram of pure, unrefined silver, at market price. Folklore and popular culture[edit | edit source]Because of the mysticism surrounding silver's lunar associations, as well as the aesthetic qualities of the white, reflective metal that cause it to be associated with purity, silver in European folklore has long been traditionally believed to be an antidote to various maladies and mythical monsters. Notably, silver was believed to be a repellent against vampires (this primarily originates from its holy connotations; also, mirrors were originally polished silver, and as such, vampires allegedly cannot be seen in them because they have no soul) and it was also believed that a werewolf, in his bestial form, could only be killed by a weapon or bullet made of silver. This has given rise to the term "silver bullet", which is used to describe things that very effectively deal with one specific problem. In the Gospels, Jesus' disciple Judas Iscariot is infamous for having taken a bribe of silver from religious leaders in Jerusalem to turn Jesus Christ over to the Romans. In heraldry, the tincture argent, in addition to being shown as silver (this has been shown at times with real silver in official representations), can also be shown as white. Occasionally, the word "silver" is used rather than argent; sometimes this is done across-the-board, sometimes to avoid repetition of the word "argent" in blazon. Precautions[edit | edit source]Silver plays no known natural biological role in humans, and possible health effects of silver are a subject of dispute. Silver itself is not toxic but most silver salts are, and some may be carcinogenic. Silver and compounds containing silver (like colloidal silver) can be absorbed into the circulatory system and become deposited in various body tissues leading to a condition called argyria which results in a blue-grayish pigmentation of the skin, eyes, and mucous membranes. Although this condition does not harm a person's health, it is disfiguring and usually permanent. Argyria is rare, and mild forms are sometimes mistaken for cyanosis. See also[edit | edit source]
External links[edit | edit source]
References[edit | edit source]
af:Silwer am:ብር (Ag) ar:فضة ast:Plata az:Gümüş bn:রূপা zh-min-nan:Ag (goân-sò͘) be:Серабро bs:Srebro bg:Сребро ca:Plata cv:Кĕмĕл ceb:Silver cs:Stříbro co:Argentu za:Ngaenz cy:Arian (elfen) da:Sølv de:Silber et:Hõbe el:Άργυρος eo:Arĝento eu:Zilar fa:نقره fur:Arint ga:Airgead (dúil) gd:Airgead (meatailt) gl:Prata (elemento) gu:ચાંદી ko:은 hy:Արծաթ hi:रूप्यम् hr:Srebro io:Arjento bpy:প্রাটা id:Perak is:Silfur it:Argento he:כסף (יסוד) pam:Pilak ka:ვერცხლი sw:Agenti (fedha) ht:Ajan ku:Zîv la:Argentum lv:Sudrabs lb:Sëlwer lt:Sidabras li:Zèlver jbo:rijno hu:Ezüst ml:വെള്ളി mi:Kawata mr:चांदी ms:Perak nah:Iztac teōcuitlatl nl:Zilver no:Sølv nn:Sølv oc:Argent (metal) uz:Kumush nds:Sülver ksh:Silber qu:Qullqi q'illay sq:Argjendi scn:Argentu simple:Silver sk:Striebro sl:Srebro sr:Сребро sh:Srebro fi:Hopea sv:Silver ta:வெள்ளி (உலோகம்) tt:Kömeş te:వెండి th:เงิน (ธาตุ) tg:Нуқра uk:Срібло ur:چاندی vec:Arxento yi:זילבער zh-yue:銀 |
|---|
 KSF
KSF