Statin
 From Wikidoc - Reading time: 12 min
From Wikidoc - Reading time: 12 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-in-Chief:: Elliot B. Tapper, MD. Department of Medicine, Beth Israel Deaconess Medical Centre
Overview[edit | edit source]
The statins (or HMG-CoA reductase inhibitors) form a class of hypolipidemic agents, used as pharmaceutical agents to lower cholesterol levels in people with or at risk of cardiovascular disease. They lower cholesterol by inhibiting the enzyme HMG-CoA reductase, which is the rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis. Inhibition of this enzyme in the liver stimulates LDL receptors, resulting in an increased clearance of low-density lipoprotein (LDL) from the bloodstream and a decrease in blood cholesterol levels. The first results can be seen after one week of use and the effect is maximal after four to six weeks.
History[edit | edit source]
Akira Endo and Masao Kuroda of Tokyo, Japan commenced research into inhibitors of HMG-CoA reductase in 1971 (Endo 1992). This team reasoned that certain microorganisms may produce inhibitors of the enzyme to defend themselves against other organisms, as mevalonate is a precursor of many substances required by organisms for the maintenance of their cell wall (ergosterol) or cytoskeleton (isoprenoids).[1]
The first agent isolated was mevastatin (ML-236B), a molecule produced by Penicillium citrinum. The pharmaceutical company Merck & Co. showed an interest in the Japanese research in 1976, and isolated lovastatin (mevinolin, MK803), the first commercially marketed statin, from the mold Aspergillus terreus. Dr Endo was awarded the 2006 Japan Prize for his work on the development of statins.
Members[edit | edit source]
The statins are divided into two groups: fermentation-derived and synthetic.
The statins include, in alphabetical order (brand names vary in different countries):
| Statin | Brand name | Derivation |
| Atorvastatin | Lipitor, Torvast | Synthetic |
| Cerivastatin | Lipobay, Baycol. (Withdrawn from the market in August, 2001 due to risk of serious adverse effects) | Synthetic |
| Fluvastatin | Lescol, Lescol XL | Synthetic |
| Lovastatin | Mevacor, Altocor | Fermentation-derived |
| Mevastatin | - | Naturally-occurring compound. Found in red yeast rice. |
| Pitavastatin | Livalo, Pitava | Synthetic |
| Pravastatin | Pravachol, Selektine, Lipostat | Fermentation-derived |
| Rosuvastatin | Crestor | Synthetic |
| Simvastatin | Zocor, Lipex | Fermentation-derived. (Simvastatin is a synthetic derivate of a fermentation product) |
| Simvastatin+Ezetimibe | Vytorin | Combination therapy |
| Lovastatin+Niacin extended-release | Advicor | Combination therapy |
| Atorvastatin+Amlodipine Besylate | Caduet | Combination therapy - Cholesterol+Blood Pressure |
LDL-lowering potency varies between agents. Cerivastatin is the most potent, followed by (in order of decreasing potency) rosuvastatin, atorvastatin, simvastatin, lovastatin, pravastatin, and fluvastatin.[2] The relative potency of pitavastatin has not yet been fully established.
Mode of action[edit | edit source]
Cholesterol lowering[edit | edit source]
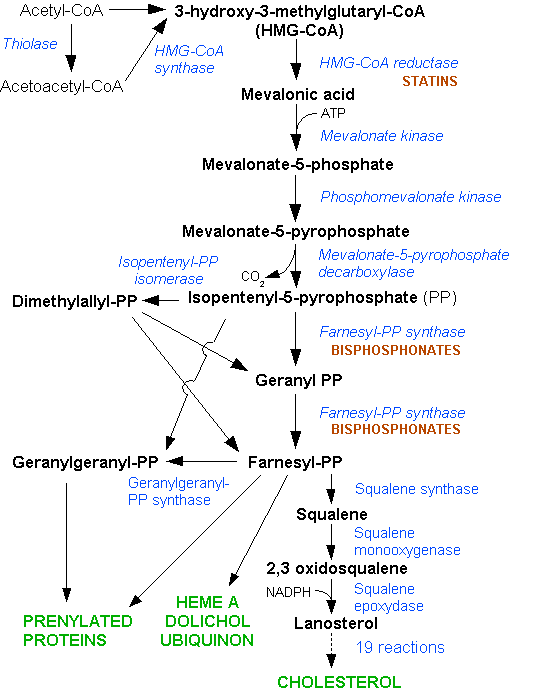
Most circulating cholesterol is manufactured internally, in amounts of about 1000 mg/day, via carbohydrate metabolism through the HMG-CoA reductase pathway. Cholesterol, both from dietary intake and secreted into the duodenum as bile from the liver, is typically absorbed at a rate of 50% by the small intestines. The typical diet in the United States and many other Western countries is estimated as adding about 200-300 mg/day to intestinal intake, an amount much smaller than that secreted into the intestine in the bile. Thus internal production is an important factor.
Cholesterol is not water-soluble, and is therefore carried in the blood in the form of lipoproteins, the type being determined by the apoprotein, a protein coating that acts as an emulsifier. The relative balance between these lipoproteins is determined by various factors, including genetics, diet, and insulin resistance. Low density lipoprotein (LDL) and very low density lipoprotein (VLDL) carry cholesterol toward tissues, and elevated levels of these lipoproteins are associated with atheroma formation (fat-containing deposits in the arterial wall) and cardiovascular disease. High density lipoprotein, in contrast, carries cholesterol back to the liver and is associated with protection against cardiovascular disease.
Statins act by competitively inhibiting HMG-CoA reductase, the first committed enzyme of the HMG-CoA reductase pathway. By reducing intracellular cholesterol levels, they cause liver cells to make more LDL receptors, leading to increased clearance of low-density lipoprotein from the bloodstream.[3]
Direct evidence of the action of statin-based cholesterol lowering on atherosclerosis was presented in the ASTEROID trial, which demonstrated regression of atheroma employing intravascular ultrasound.[4]
[edit | edit source]
Statins exhibit action beyond lipid-lowering activity in the prevention of atherosclerosis. Researchers hypothesize that statins prevent cardiovascular disease via four proposed mechanisms (all subjects of a large body of biomedical research):[5]
- Improving endothelial function
- Modulate inflammatory responses
- Maintain plaque stability
- Prevent thrombus formation
Indications and uses[edit | edit source]
Statins, the most potent cholesterol-lowering agents available, lower LDL cholesterol (so-called "bad cholesterol") by 30–50%.[6] However, they have less effect than the fibrates or niacin in reducing triglycerides and raising HDL-cholesterol ("good cholesterol"). Professional guidelines generally require that the patient has tried a cholesterol-lowering diet before statin use is considered; statins or other pharmacologic agents may then be recommended for patients who do not meet their lipid-lowering goals through diet and lifestyle approaches.
The indications for the prescription of statins have broadened over the years. Initial studies, such as the Scandinavian Simvastatin Survival Study (4S), supported the use of statins in secondary prevention for cardiovascular disease, or as primary prevention only when the risk for cardiovascular disease was significantly raised (as indicated by the Framingham risk score).[7] Indications were broadened considerably by studies such as the Heart Protection Study (HPS), which showed preventative effects of statin use in specific risk groups, such as diabetics. The ASTEROID trial, published in 2006, using only a statin at high dose, achieved lower than usual target calculated LDL values and showed disease regression within the coronary arteries using intravascular ultrasonography.[4]
Based on clinical trials, the National Cholesterol Education Program guidelines, and the increasing focus on aggressively lowering LDL-cholesterol, the statins continue to play an important role in both the primary and secondary prevention of coronary heart disease, myocardial infarction, stroke and peripheral artery disease.
Research continues into other areas where statins also appear to have a favorable effect: inflammation, dementia,[8] cancer,[9] nuclear cataracts,[10] and pulmonary hypertension.
A Trial Based Approach to Statin Guidelines[edit | edit source]
Shown below is a table suggesting an approach to the use of statin according to different trials.[11]
| Patient Group | Supporting Trials |
| Secondary prevention | |
| High-quality randomized clinical trial data support the use of statin therapy as an effective adjunct to diet, exercise, and smoking cessation for secondary prevention patients with a history of myocardial infarction, stroke, or clinically apparent atherosclerosis. | 4S, CARE, LIPID, PROSPER, HPS |
| High-quality randomized clinical trial data in secondary prevention support maximizing the intensity of statin treatment and maintaining compliance with the treatment regimen. | PROVE-IT, TNT, IDEAL, A to Z, SEARCH |
| Primary prevention | |
| High-quality randomized clinical trial data support the use of statin therapy as an effective adjunct to diet, exercise, and smoking cessation in the setting of primary prevention for middle-aged and older individuals with elevated low-density lipoprotein cholesterol, low high-density lipoprotein cholesterol, elevated C-reactive protein, or multiple risk factors inclusive of diabetes and hypertension. | WOSCOPS, MEGA, AFCAPS/TexCAPS, CARDS, ASCOT-LLA, JUPITER |
| Heart failure and renal insufficiency: | |
| High-quality randomized clinical trial data do not support the use of statin monotherapy for adults with isolated heart failure or those underlying hemodialysis. | CORONA, GISSI-HF, 4-D, AURORA |
| High-quality randomized clinical trial data support the use of statin therapy among adults with chronic renal insufficiency, at least when given in combination with ezetimib. | SHARP |
| Other conditions: | |
| For adults who do not meet the above criteria, physicians may consider issues such as genetic predisposition or a strong family history of premature coronary disease when making decisions for individual patient. For some patients, such as those suspected of having familial hyperlipidemia, referral to lipid or atherosclerosis specialists may be useful for consideration of further evaluation and the use of statins despite the absence of hard end point trial data. |
None-clinical judgement, expert opinion |
Pharmacogenomics[edit | edit source]
A 2004 study showed that patients with one of two common single nucleotide polymorphisms (small genetic variations) in the HMG-CoA reductase gene were less responsive to statins.[12]
Safety[edit | edit source]
While some patients on statin therapy report myalgias, muscle cramps, or far less-frequent gastrointestinal or other symptoms, similar symptoms are also reported with placebo use in all the large statin safety/efficacy trials and usually resolve, either on their own or on temporarily lowering/stopping the dose.
Hepatic Injury[edit | edit source]
While clinically important hepatotoxicity is exceedingly rare, statin hepatopathy is more common and ranges from mild liver enzyme derangements to, rarely, liver failure. The first response to liver enzyme elevation in the setting of statin use ought to be to rule out other causes.[13].
Any drug-induced hepatopathy is defined as an elevation in the alanine aminotransferase (ALT) levels of greater than 3-times the upper limit of normal or elvation of indirect bilirubin levels of greater than 2 times the upper limit of normal. With respect to statins, the liver injury is purely hepatocellular - a 'transaminitis' - and present as a dose-dependent, class-effect in 1-3% of patients taking statins, as observed in clinical trials (though often not significantly different from transaminase elevations in the placebo groups).[14][15][16] Generally - in more than 70% of instances[17] - these elevations return to normal over time. Rarely, this injury becomes chronic. If patients are to experience a clinically significant liver injury from statin use, it typically manifests itself within 9-11 weeks.[18] Liver failure is the rarest side-effect; 3 patients have required liver transplantation (though 2 were taking the long-discontinued cerivistatin).[19]
Myopathy[edit | edit source]
Statin induced myopathy is common while serious muscle damage is rare. Many patients complain of muscle pain without laboratory evidence of muscle damage. [20] The chief concern, however, is frank muscle breakdown or rhabdomyolysis which can leaded to renal failure, though it is very rare. One 2004 study found that of 10,000 patients treated for one year, 0.44 will develop rhabdomyolysis. Cerivastatin, which was withdrawn by its manufacturer for this reason in 2001, had a much higher incidence (more than 10x).[21] All commonly used statins show somewhat similar results, however the newer statins, characterized by longer pharmacological half-lives and more cellular specificity, have had a better ratio of efficacy to lower adverse effect rates. In a review of the randomized-controlled trials for statin therapy, 7 patients experienced rhabdomyolysis while receiving statins at the same time as 5 patients who were receiving placebo.[22] Between 1990 and 2002, the Food and Drug Administration received 3339 reports of patients with rhabdomyolysis while taking a statin, 10.4% of which were life-threatening. However, 52% of these patients were taking other medications associated with rhabdomyolisis. [23]
The pathophysiology of statin-myopathy is fairly well understood. On one hand, Co-Enzyme Q-10 (ubiquinone) levels are decreased in statin use;[24] Ubiquinoine is a product of the steroid synthesis pathways and as such its production is reduced in statin use. It is also a key component of the electron transport chain of mitochondria. Accordingly, with less ubiquinone, the cell is less likely to meet its energy needs and may die. Coenzyme Q10 supplements are sometimes used to treat statin-associated myopathy, though evidence of their effectiveness is currently lacking.[25] Additionally, statins - and not their metabolites - are directly toxic to liver cells. They have been shown to increase cell-death by causing both an increase in intracellular calcium concentration and the translocation into the mitochondria of bax, a pro-apoptotic protein.[26]
The risk of myopathy may be lowest with pravastatin and fluvastatin probably because they are less lipophillic and as a result have less muscle penetration.
N-of-1 trials have examined this:
Neuromuscular Disorders and Mitochrondrial Myopathies[edit | edit source]
There is also a small, but growing literature of statin use 'unmasking' latent neurmuscular disorders and mitochondrial myopathies such as McArdle's Disease, Kennedy's Disease, MELAS, and Myasthenia Gravis.[32][33]
Memory Loss[edit | edit source]
Memory loss have been reported by several patients taking statins, which have increased concerns about the effect of statins on the cognitive function. Statins, mainly the lipophilic types, might cause small decrease in memory and attention but there is no certainty regarding the clinical significance of this side effect nor the long term effect of statin on memory.[34][35]
Cancer Concerns[edit | edit source]
Despite initial concerns that statins might increase the risk of cancer, various studies concluded later that statins have no influence on cancer risk (including the heart protection study and a 2006 meta-analysis[36]). Indeed, a 2005 trial showed that patients taking statins for over 5 years reduced their risk of colorectal cancer by 50%; this effect was not exhibited by fibrates. The trialists warn that the number needed to treat would approximate 5000, making statins unlikely tools for primary prevention.[37]
Drug interactions[edit | edit source]
Combining any statin with a fibrate, another category of lipid-lowering drugs, increases the risks for rhabdomyolysis to almost 6.0 per 10,000 person-years.[21] Most physicians have now abandoned routine monitoring of liver enzymes and creatine kinase, although they still consider this prudent in those on high-dose statins or in those on statin/fibrate combinations, and mandatory in the case of muscle cramps or of deterioration in renal function.
Consumption of grapefruit or grapefruit juice inhibits the metabolism of statins—furanocoumarins in grapefruit juice inhibit the cytochrome P450 enzyme CYP3A4, which is involved in the metabolism of most statins (however it is a major inhibitor of only atorvastatin, lovastatin and simvastatin) and some other medications[38] (it had been thought that flavonoids were responsible). This increases the levels of the statin, increasing the risk of dose-related adverse effects (including myopathy/rhabdomyolysis). Consequently, consumption of grapefruit juice is not recommended in patients undergoing therapy with most statins. An alternative, somewhat risky, approach is that some users take grapefruit juice to enhance the effect of lower (hence cheaper) doses of statins. This is not recommended as a result of the increased risk and potential for statin toxicity.
Controversy[edit | edit source]
Some scientists take a skeptical view of the need for many people to require statin treatment. The International Network of Cholesterol Skeptics is a group that has questioned the "lipid hypothesis" that supports cholesterol lowering as a preventive measure for heart disease, and has argued that statins - especially at higher doses - may not be as beneficial or safe as suggested.[39] Similarly, some authors argue that recommendations for the expanded use of statins to stave off cardiovascular disease are not supported by evidence.[40]
References[edit | edit source]
- ↑ Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res 1992;33:1569-82. PMID 1464741.
- ↑ Shepherd J, Hunninghake DB, Barter P, McKenney JM, Hutchinson HG (2003). "Guidelines for lowering lipids to reduce coronary artery disease risk: a comparison of rosuvastatin with atorvastatin, pravastatin, and simvastatin for achieving lipid-lowering goals". Am. J. Cardiol. 91 (5A): 11C–17C, discussion 17C-19C. PMID 12646338.
- ↑ Ma PT, Gil G, Sudhof TC, Bilheimer DW, Goldstein JL, Brown MS. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc Natl Acad Sci U S A 1986;83:8370-4. PMID 3464957.
- ↑ 4.0 4.1 Nissen S, Nicholls S, Sipahi I, Libby P, Raichlen J, Ballantyne C, Davignon J, Erbel R, Fruchart J, Tardif J, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu E (2006). "Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial". JAMA. 295 (13): 1556–65. PMID 16533939.
- ↑ Furberg, Curt D. (1999). "Natural Statins and Stroke Risk". Circulation. American Heart Association. 99: 185–188.
- ↑ Jones P, Kafonek S, Laurora I, Hunninghake D (1998). "Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study)". Am J Cardiol. 81 (5): 582–7. PMID 9514454.
- ↑ Wilson P, D'Agostino R, Levy D, Belanger A, Silbershatz H, Kannel W (1998). "Prediction of coronary heart disease using risk factor categories". Circulation. 97 (18): 1837–47. PMID 9603539.
- ↑ Wolozin, B (July 19, 2007). "Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease". BMC Medicine. 5: 20. doi:10.1186/1741-7015-5-20. PMID 17640385. Unknown parameter
|coauthors=ignored (help) - ↑ Khurana, V (May 2007). "Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans". Chest. 131 (5): 1282–1288. PMID 17494779. Unknown parameter
|coauthors=ignored (help) - ↑ Klein, Barbara E. K., MD, MPH (2006). "Statin Use and Incident Nuclear Cataract". Journal of the American Medical Association. 295 (23): 2752–8. PMID 16788130. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Ridker P, Wilson PF. A Trial-Based Approach to Statin Guidelines. JAMA. 2013;():-. doi:10.1001/jama.2013.276529.
- ↑ Chasman DI, Posada D, Subrahmanyan L, Cook NR, Stanton VP Jr, Ridker PM. Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA 2004;291:2821-7. PMID 15199031.
- ↑ Bhardwaj and Chalasani. Lipid lowering agents that cause drug induced hepatotoxicity. Clin Liv Dis 2007;11(3):662-9.
- ↑ Am J Cardiol 2006;97[suppl]:77C–81C
- ↑ De Denus S et al. Statin and liver toxicity: a meta-analysis. Pharmacotherapy 2004;24(5):584-91
- ↑ Calderon RM et al. Statins in the Treatment of Dyslipidema in the Presence of Elevated Liver Aminotransferase Levels: A Therapeutic Dilemma. Mayo Clin Proc 2010;85(4):349-56
- ↑ Bays H. Statin Safety: an overview and assessment of the data-2005. Am J Card 2006;97(8A):6C-26C
- ↑ Hepatology 2006;44:1581-1588
- ↑ Liver Transpl 2004;10:1018 –1023
- ↑ Phillips PS. Ann Intern Med 2002;137:581–5.
- ↑ 21.0 21.1 Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, Platt R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004;292:2585-90. PMID 15572716.
- ↑ JAMA 2003;289:1681–1690
- ↑ Am J Cardiol 2006;97[suppl]:69C–76C
- ↑ Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L, Caputo S, Greco A, Littarru G (1993). "Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study". J Clin Pharmacol. 33 (3): 226–9. PMID 8463436.
- ↑ Marcoff L, Thompson PD (2007). "The role of coenzyme Q10 in statin-associated myopathy: a systematic review". J. Am. Coll. Cardiol. 49 (23): 2231–7. doi:10.1016/j.jacc.2007.02.049. PMID 17560286.
- ↑ JPET 314:1032–1041, 2005
- ↑ Herrett E, Williamson E, Brack K, Beaumont D, Perkins A, Thayne A; et al. (2021). "Statin treatment and muscle symptoms: series of randomised, placebo controlled n-of-1 trials". BMJ. 372: n135. doi:10.1136/bmj.n135. PMC 7903384 Check
|pmc=value (help). PMID 33627334 Check|pmid=value (help). - ↑ Herrett E, Williamson E, Brack K, Perkins A, Thayne A, Shakur-Still H; et al. (2021). "The effect of statins on muscle symptoms in primary care: the StatinWISE series of 200 N-of-1 RCTs". Health Technol Assess. 25 (16): 1–62. doi:10.3310/hta25160. PMC 8020196 Check
|pmc=value (help). PMID 33709907 Check|pmid=value (help). - ↑ Howard JP, Wood FA, Finegold JA, Nowbar AN, Thompson DM, Arnold AD; et al. (2021). "Side Effect Patterns in a Crossover Trial of Statin, Placebo, and No Treatment". J Am Coll Cardiol. 78 (12): 1210–1222. doi:10.1016/j.jacc.2021.07.022. PMC 8453640 Check
|pmc=value (help). PMID 34531021 Check|pmid=value (help). - ↑ Wood FA, Howard JP, Finegold JA, Nowbar AN, Thompson DM, Arnold AD; et al. (2020). "N-of-1 Trial of a Statin, Placebo, or No Treatment to Assess Side Effects". N Engl J Med. 383 (22): 2182–2184. doi:10.1056/NEJMc2031173. PMID 33196154 Check
|pmid=value (help). - ↑ Tudor K, Brooks J, Howick J, Fox R, Aveyard P (2020). "Tackling statin intolerance with n-of-1 trials (TaSINI) in primary care: protocol for a feasibility randomised trial to increase statin adherence". BMJ Open. 10 (2): e033070. doi:10.1136/bmjopen-2019-033070. PMC 7044821 Check
|pmc=value (help). PMID 32051312 Check|pmid=value (help). - ↑ Medicine 2006;85:82–85.
- ↑ Arch Intern Med. 2006;166:1519-1524
- ↑ Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA; et al. (2000). "Effects of lovastatin on cognitive function and psychological well-being". Am J Med. 108 (7): 538–46. PMID 10806282.
- ↑ Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB (2004). "Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults". Am J Med. 117 (11): 823–9. doi:10.1016/j.amjmed.2004.07.041. PMID 15589485.
- ↑ Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA 2006;295:74-80. PMID 16391219.
- ↑ Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med 2005;352:2184-92. PMID 15917383.
- ↑ Kane GC, Lipsky JJ. Drug-grapefruit juice interactions. Mayo Clin Proc 2000;75:933-42. PMID 10994829.
- ↑ Ravnskov U, Rosch P, Sutter M, Houston M (2006). "Should we lower cholesterol as much as possible?". BMJ. 332 (7553): 1330–2. PMID 16740566.
- ↑ Abramson J, Wright J (2007). "Are lipid-lowering guidelines evidence-based?". Lancet. 369 (9557): 168–9. PMID 17240267.
External links[edit | edit source]
- Statin page at Bandolier, an evidence-based medicine journal
 KSF
KSF