Telbivudine description
 From Wikidoc - Reading time: 2 min
From Wikidoc - Reading time: 2 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Description[edit | edit source]
Tyzeka is the trade name for telbivudine, a synthetic thymidine nucleoside analogue with activity against hepatitis B virus (HBV). The chemical name for telbivudine is 1-((2S,4R,5S)-4-hydroxy-5-hydroxymethyltetrahydrofuran-2-y1)-5-methyl-1H-pyrimidine-2,4-dione, or 1-(2-deoxy-β-L-ribofuranosyl)-5-methyluracil. Telbivudine is the unmodified β-L enantiomer of the naturally occurring nucleoside, thymidine. Its molecular formula is C10H14N2O5, which corresponds to a molecular weight of 242.23. Telbivudine has the following structural formula:
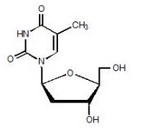 |
Telbivudine is a white to slightly yellowish powder. Telbivudine is sparingly soluble in water (greater than 20 mg per mL), and very slightly soluble in absolute ethanol (0.7 mg per mL) and n-octanol (0.1 mg per mL).
Tyzeka film-coated tablets are available for oral administration in 600 mg strength. Tyzeka 600 mg film-coated tablets contain the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, povidone, and sodium starch glycolate. The tablet coating contains titanium dioxide, polyethylene glycol, talc, and hypromellose.
Tyzeka oral solution is available for oral administration in 100 mg per 5 mL strength. Tyzeka oral solution contains the following inactive ingredients: citric acid anhydrous, benzoic acid, passion fruit flavor, sodium saccharin, sodium hydroxide, and purified water. A 600 mg dose (30 mL) of Tyzeka oral solution contains approximately 47 mg of sodium.[1]
References[edit | edit source]
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022011s013lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.
 KSF
KSF