Teuber reaction
 From Wikidoc - Reading time: 2 min
From Wikidoc - Reading time: 2 min
|
WikiDoc Resources for Teuber reaction |
|
Articles |
|---|
|
Most recent articles on Teuber reaction Most cited articles on Teuber reaction |
|
Media |
|
Powerpoint slides on Teuber reaction |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Teuber reaction at Clinical Trials.gov Trial results on Teuber reaction Clinical Trials on Teuber reaction at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Teuber reaction NICE Guidance on Teuber reaction
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Teuber reaction Discussion groups on Teuber reaction Patient Handouts on Teuber reaction Directions to Hospitals Treating Teuber reaction Risk calculators and risk factors for Teuber reaction
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Teuber reaction |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
The Teuber reaction is the selective oxidation of phenols and aromatic amines to quinones. [1]
Useful oxidizing reagents include nitrosodisulfonate salts, especially Fremy's salt, a dipotassium salt [2] or oxone [3].
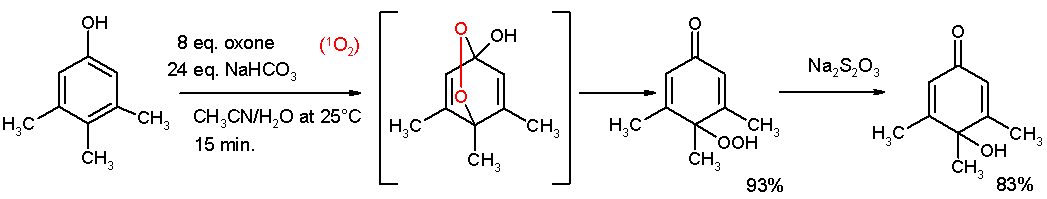
References[edit | edit source]
- ↑ 2,5-Cyclohexadiene-1,4-dione, 2,3,5-trimethyl Organic Syntheses, Coll. Vol. 6, p.1010 (1988); Vol. 52, p.83 (1972).
- ↑ 2,5-Cyclohexadiene-1,4-dione, 2,3,5-trimethyl Organic Syntheses, Coll. Vol. 6, p.1010 (1988); Vol. 52, p.83 (1972).
- ↑ Oxidative De-aromatization of para-Alkyl Phenols into para-Peroxyquinols and para-Quinols Mediated by Oxone as a Source of Singlet Oxygen M. Carmen Carreño, Marcos González-López, Antonio Urbano Angewandte Chemie International Edition Volume 45, Issue 17 , Pages 2737 - 2741 2006
 KSF
KSF