Transduction (biophysics)
 From Wikidoc - Reading time: 16 min
From Wikidoc - Reading time: 16 min
Editor-In-Chief: Henry A. Hoff
In biophysics, transduction is the conveyance of energy from one electron (a donor) to another (a receptor), at the same time that the class of energy changes.
Photonic energy, the kinetic energy of a photon, when interacting with an electron, may follow these paths:
- stimulated emission as a photon of less energy;
- transference to a recipient with no change in class;
- dissipation as heat; or
- transductance.
Microwave radiation transduction[edit | edit source]
Trace amounts of biogenic magnetite occur in animal and human tissues.[1] Microwaves between 0.5 and 10 GHz are readily absorbed by magnetite.[1] Energy absorbed by magnetite is transduced into acoustic vibration which may be dissipated to cellular structures in close proximity.[1]
The total concentration of magnetite in brain tissues is ~5-100 ppb.[1]
Infrared radiation transduction[edit | edit source]
Infrared radiation (IR) covers a range from roughly 300 GHz (1 mm) to 400 THz (750 nm). Far-infrared (IRC), 300 GHz to 30 THz (10 µm), is typically absorbed by molecular motions in liquids, usually water. The mid-infrared (IRB), 30 to 120 THz (2.5 µm), is absorbed by molecular vibrations. The near-infrared (IRA), 120 to 400 THz, affects physical processes similar to those for visible light.
More than half of the solar energy that reaches human skin is IR.[2] IRB and IRC do not penetrate deeply into the skin, but more than 65% of IRA reaches the dermis.[2] IRA induces gene transcription.[3] IRA significantly contributes to extrinsic skin aging.[2] In human skin, infrared radiation induces matrix metalloproteinase, an initial signaling step in near-infrared (700 nm to 1400 nm) radiation-induced signal transduction.[4]
Photosynthesis[edit | edit source]
In photosynthesis, when the electrons of the "chlorophyll pair" receive the photon energy from the "collecting" associated pigments, the photonic energy is "destined" to link one molecule of phosphate to one of NAD. The resulting NADP in turn will use the stored energy in the generation of ATP, which is the end point of the light-induced photosynthetic process. This means that the photon's energy ends up its circuit by being transduced to an electron that takes part in the formation of a molecular link of energy-rich phosphate.
In the pathway of this end-point transduction, the energy is transferred along a number of molecules (cytochromes), in a downward way so that energy is partially dissipated at each step. The liberated heat energy serves the homeostasis of the plant, and at the end of the chain the remaining energy is perhaps exactly the one that is needed to build NADP.
This process is committed; i.e. there is no return path. Homeostasis, theoretically, might save the day only at the beginning: before the luminic energy transferred to the "chlorophyl pair" is conveyed to the first element of the cytochrome chain, there is a gap in the process when the energy is carried as a series of excitons. These are now called resonant-energy-transferring molecules of the chlorophyll class, which transfer what is considered electromagnetic energy, from one to its neighbor with no participation of electrons nor enzymes. At this stage, if the first pigment has received an excess of light, the "exciton" perhaps might dissipate the energy as heat.
Phototransduction[edit | edit source]
Visual phototransduction is a process by which light is converted into electrical signals in photoreceptors such as the rod cells, cone cells and photosensitive ganglion cells of the retina of the eye. The rods and cones contain a chromophore (11-cis-retinal, the aldehyde of Vitamin A1 and light-absorbing portion) bound to a cell membrane protein, opsin. Rods deal with low light level. Cones can code the colour of an image through comparison of the outputs of the three different types of cones. Each cone type responds best to certain wavelengths, or colours, of light because each type has a slightly different opsin. The three types of cones are L-cones, M-cones and S-cones that respond optimally to long wavelengths (reddish colour), medium wavelengths (greenish colour), and short wavelengths (bluish colour) respectively.
There is an ongoing outward potassium current through nongated K+-selective channels. This outward current tends to hyperpolarise the photoreceptor at around -70 mV (the equilibrium potential for K+). There is also an inward sodium current carried by cGMP-gated sodium channels. This so-called 'dark current' depolarises the cell to around -40 mV. Note that this is significantly more depolarised than most other neurons. A high density of Na+-K+ pumps enables the photoreceptor to maintain a steady intracellular concentration of Na+ and K+.
In the dark[edit | edit source]
Photoreceptors are depolarized in the dark. Light hyperpolarizes and switches these cells off. Once switched off the next cell is activated and sends an excitatory signal down the neural pathway. In the dark, cGMP levels are high and keep cGMP-gated sodium channels open allowing a steady inward current, called the dark current. This dark current keeps the cell depolarised at about -40 mV.
The depolarisation of the cell membrane opens voltage-gated calcium channels. An increased intracellular concentration of Ca+ causes vesicles containing special chemicals, called neurotransmitters, to merge with the cell membrane, therefore releasing the neurotransmitter into the synaptic cleft, an area between the end of one cell and the beginning of another neuron. The neurotransmitter released is glutamate, an excitatory neurotransmitter.
In the cone pathway glutamate:
- Hyperpolarizes on-center bipolar cells. Glutamate that is released from the photoreceptors in the dark binds to metabotropic glutamate receptors (mGluR6), which, through a G-protein coupling mechanism, causes non-specific cation channels in the cells to close, thus hyperpolarizing the bipolar cell.
- Depolarizes off-center bipolar cells. Binding of glutamate to ionotropic glutamate receptors results in an inward cation current that depolarizes the bipolar cell.
In the light[edit | edit source]
- A light photon interacts with the retinal in a photoreceptor. The retinal undergoes isomerisation, changing from the 11-cis to all-trans configuration.
- Retinal no longer fits into the opsin binding site.
- Opsin therefore undergoes a conformational change to metarhodopsin II.
- Metarhodopsin II is unstable and splits, yielding opsin and all-trans retinal.
- The opsin activates the regulatory protein transducin. This causes transducin to dissociate from its bound GDP, and bind GTP, then the alpha subunit of transducin dissociates from the beta and gamma subunits, with the GTP still bound to the alpha subunit.
- The alpha subunit-GTP complex activates phosphodiesterase.
- Phosphodiesterase breaks down cGMP to 5'-GMP. This lowers the concentration of cGMP and therefore the sodium channels close.
- Closure of the sodium channels causes hyperpolarisation of the cell due to the ongoing potassium current.
- Hyperpolarisation of the photoreceptor results in a decrease in the amount of the neurotransmitter glutamate that is released by the cell.
- A decrease in the amount of glutamate released by the photoreceptors causes depolarization of On center bipolar cells (rod and cone On bipolar cells) and hyperpolarization of cone Off bipolar cells.
Deactivation of the phototransduction cascade[edit | edit source]
GTPase Activating Protein (GAP) interacts with the alpha subunit of transducin, and causes it to hydrolyse its bound GTP to GDP, and thus halts the action of phosphodiesterase, stopping the transformation of cGMP to GMP.
Guanylate Cyclase Activating Protein (GCAP) is a calcium binding protein, and as the calcium levels in the cell have decreased, GCAP dissociates from its bound calcium ions, and interacts with Guanylate Cyclase, activating it. Guanylate Cyclase then proceeds to transform GTP to cGMP, replenishing the cell's cGMP levels and thus reopening the sodium channels that were closed during phototransduction.
Finally, Metarhodopsin II is deactivated. Recoverin, another calcium binding protein, is normally bound to Rhodopsin Kinase when calcium is present. When the calcium levels fall during phototransduction, the calcium dissociates from recoverin, and rhodopsin kinase is released, when it proceeds to phosphorylate metarhodopsin II, which decreases its affinity for transducin. Finally, arrestin, another protein, binds the phosphorylated metarhodopsin II, completely deactivating it. Thus, finally, phototransduction is deactivated, and the dark current and glutamate release is restored. It is this pathway, where Metarhodopsin II is phosphorylated and bound to arrestin and thus deactivated, which is thought to be responsible for the S2 component of dark adaptation. The S2 component represents a linear section of the dark adaptation function present at the beginning of dark adaptation for all bleaching intensities.
All-trans retinal is transported to the pigment epithelial cells to be reduced to all-trans retinol, the precursor to 11-cis retinal. This is then transported back to the rods. All-trans retinol cannot be synthesised by humans and must be supplied by vitamin A in the diet. Deficiency of all-trans retinol can lead to night blindness. This is part of the bleach and recycle process of retinoids in the photoreceptors and retinal pigment epithelium.
Ultraviolet transduction[edit | edit source]
Ultraviolet radiation (UV) can affect DNA, and cytoplasmic and membrane structures. UV can directly affect cytoplasmically located transcription factors, kinases closely located to the cell membrane, and membrane receptors.[5] These targets transduce the UV signal by various mechanisms. UV can interfere with cytokine signaling and induce apoptosis.[5]
X-ray transduction[edit | edit source]
In x-ray imaging systems, the energy contained in the x-ray intensity distribution is converted into digital electronic form. Intensifying screens transduce the x-ray energy into optical energy and image intensifiers transduce the optical energy into electron beam energy. The electron beam energy is subsequently transduced back into optical energy at the output phosphor.[6]
The Swank factor is a critical performance parameter of an imaging detector for the degradation of the signal to noise ratio due to variations in the detector response. It can be evaluated by the absorption of monoenergetic x-ray photons using measured pulse height spectra.
The Swank factor for x-ray imaging using amorphous selenium at mammographic energies that lie close to the K-absorption edge of selenium has been found to be very close to the maximum value of unity for x-ray transduction.[7]
Although X-radiation is used to treat a lot of cancers, it also produces side effects in normal tissue such as radiodermatitis. In response to this, certain genes may transduce this energy to prevent X-ray induced apoptosis in some cells.[8]
Gamma ray transduction[edit | edit source]
Gamma rays consist of photons with energies above about 100 keV. Hard X-rays overlap the range of lower energy gamma rays. The distinction between the two terms depends on the source of the radiation, not its wavelength. X-ray photons are generated by energetic electron processes, gamma rays by transitions within atomic nuclei. In passing through tissue, gamma radiation ionizes by the photoelectric effect, Compton scattering, and pair production. Each mechanism dominates over a particular energy range: photoelectric effect - below 50 keV, Compton scattering - 100 keV to 10 MeV, pair production - 1 MeV and up. During lightning, gamma rays are generated which escape the Earth's atmosphere.[9] These occur ~50 times per day globally with energies exceeding 20 MeV. The gamma radiation may fountain up from the tops of high thunderclouds and irradiate spacecraft.
Like X-rays, gamma rays are a form of ionizing radiation that can damage living tissue. Indeed, gamma radiation can induce apoptosis. The same targets transducing signals in the cell that ultimately converge to cause apoptosis are also involved in gamma radiation induced apoptosis.[10]
Gamma rays are not stopped by the skin. They can induce DNA alteration by interfering with the genetic material of the cell. DNA double-strand breaks are generally accepted to be the most biologically significant lesion by which ionizing radiation causes cancer and hereditary disease.[11] A study done on Russian nuclear workers exposed to external whole-body gamma radiation at high cumulative doses shows the link between radiation exposure and death from leukemia, lung, liver, skeletal and other solid cancers.[12] In addition to irradiation damage, gamma rays produce thermal burn injuries and induce an immunosuppressive effect.[13][14]
After gamma irradiation, and the breaking of DNA double-strands, a cell can repair the damaged genetic material to the limit of its capability. The repair process works well after high-dose exposure but is much slower in the case of a low-dose exposure.[11]
This may mean that a chronic low-dose exposure cannot be fought by the body. The probability of detecting small alterations or of a detectable defect occurring is most likely small enough that the cell would replicate before initiating a full repair. Some cells can not detect their own genetic defects.
Beta particle transduction[edit | edit source]
Beta particles are high-energy (up to a few tens of MeV), high-speed (~0.60 c) electrons or positrons that are a form of ionizing radiation. Beta-irradiation can induce apoptosis and activate apoptosis pathways by mitochondrial damage and death receptors in leukemia cells.[15]
Very fast electrons produced in quantity by fast energetic charged particles knocking orbiting electrons out of atoms are collectively called delta radiation, although the term or delta ray is rarely used today, when they have sufficient energy to ionize additional atoms through subsequent interactions on their own. These electrons are usually referred to as knock-on electrons. Delta rays that subsequently produce additional radiation (tertiary radiation) have been called epsilon radiation, which is a term very rarely used today.
Neutron radiation transduction[edit | edit source]
Neutrons may be emitted during spontaneous or induced nuclear fission, nuclear fusion processes, in cosmic ray interactions, or from other nuclear reactions such as light element fusion, e.g., a beryllium nucleus reacting with an alpha particle (helium nucleus) transforming into a carbon nucleus and emitting a neutron.
Neutron radiation is often called indirectly ionizing radiation. It does not ionize atoms in the same way protons, photons, and electrons do (exciting an electron) because neutrons have no charge. However, neutron interactions are largely ionizing, for example when neutron absorption results in gamma emission and the gamma subsequently removes an electron from an atom, or a nucleus recoiling from a neutron interaction is ionized and causes more traditional subsequent ionization in other atoms. Because neutrons are uncharged, they are more penetrating than alpha radiation or beta radiation. In some cases they are more penetrating than gamma radiation, which is impeded in materials of high atomic number. In hydrogen, a low energy neutron may not be as penetrating as a high energy gamma.
In neutron activation, neutron radiation can induce radioactivity in most substances it encounters, including body tissues. This occurs through the capture of neutrons by atomic nuclei, which are transformed to another nuclide, frequently a radionuclide. This process accounts for much of the radioactive material released by the detonation of a nuclear weapon. It is also a problem in nuclear fission and nuclear fusion installations, as it gradually renders the equipment radioactive; eventually the hardware must be replaced and disposed of as low-level radioactive waste. Neutrons readily pass through most material, but interact enough to cause biological damage. Due to the high kinetic energy of neutrons (up to 14 MeV), they are considered to be the most severe and dangerous radiation available. In living tissue, neutrons have a relatively high Relative Biological Effectiveness (RBE), and are roughly ten times more effective at causing cancers compared to photon or beta radiation of equivalent radiation exposure.
Inside a spacecraft, shielding material modifies the interaction with space radiation originating secondary radiation composed of neutrons and heavy ions.
Geomagnetically trapped particle radiation transduction[edit | edit source]
Space radiation that interacts with the Earth's geomagnetic field becomes geomagnetically trapped particle radiation. Such radiation is comprised of electrons with energies up to 7 MeV, protons with energies up to 600 MeV, and low energy heavy ions.[16]
Solar particle radiation transduction[edit | edit source]
Solar particle radiation consists of charged particles in large clouds, mainly protons with an energy of about 1 GeV.[16]
Galactic cosmic radiation transduction[edit | edit source]
Space radiation consists of geomagnetically trapped particle radiation, solar particle radiation, and galactic cosmic radiation.[16] Galactic cosmic radiation has energies between 1 and 103 GeV and consists of 87% protons, 12% alpha particles, and 1% heavy ions.[16]
Signal transduction[edit | edit source]
In biology, signal transduction refers to any process by which a cell converts one kind of signal or stimulus into another, most often involving ordered sequences of biochemical reactions inside the cell, that are carried out by enzymes and linked through second messengers resulting in what is thought of as a "second messenger pathway".
Many, if not all, of the signal conversions may convey or use energy from one electron (a donor) to another (a receptor), while the form of energy remains unchanged or usually does not change. Energy consumption occurs. But the pathway may not always be committed or leading.
A signal transduction is usually rapid, lasting on the order of milliseconds in the case of ion flux, to minutes for the activation of protein and lipid mediated kinase cascades. In many signal transduction processes, the number of proteins and other molecules participating in these events increases as the process eminates from the initial stimulus, resulting in a "signal cascade". Often a relatively small stimulus elicits a large response.
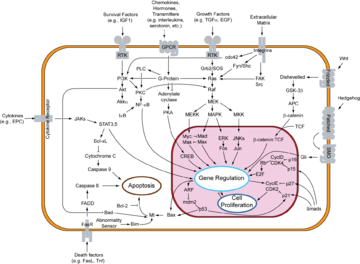
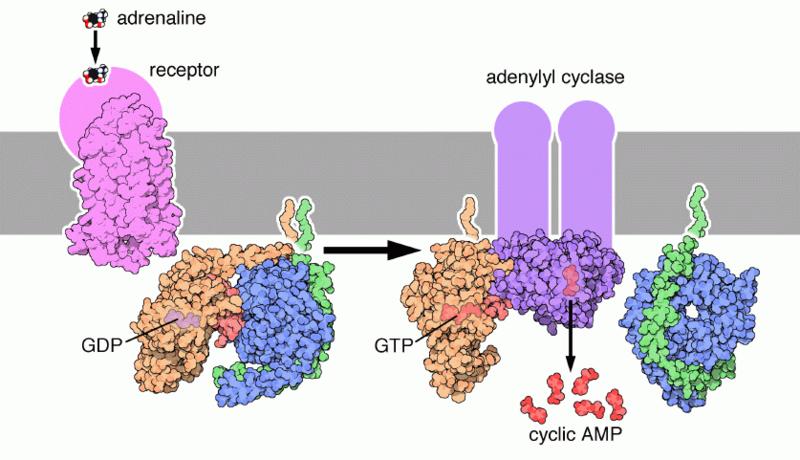
Signal transductions can occur in many directions. Some occur along the cytoplasmic surface of the cell membrane, like the epinephrin pathway.
Others occur inwardly, such as the MAPK/ERK pathway, ultimately arriving inside the cell nucleus.

If a possible set of proteins for the MAPK/ERK pathway of signal transduction were linked together physically without the proteins being unfolded, their connected length as suggested in the table below would only be ~66 nm from the cytoplasmic side of the cell membrane to the cytoplasmic side of the nuclear envelope.
| Protein | Mass (Da) | Likely diameter (nm) | Connected length (nm) |
|---|---|---|---|
| GRB2 | 25k | ~7 | ~7 |
| SOS1 | 152k | ~13 | ~20 |
| RASD1 | 32k | ~8 | ~28 |
| RAF1 | 73k | ~10 | ~38 |
| MAP3K1 | 164k | ~13 | ~51 |
| MKNK1 | 30k | ~8 | ~59 |
| CREB1 | 25k | ~7 | ~66 |
For a typical cell of diameter 10 µm, with a nucleus of about 5 µm in diameter, about 2400 nm would remain for the signal transduction to occur across (the 6 gaps). The first 5 gaps involve activation and phosphorylation. The series of kinases from RAF to MKNK1 (MNK) is a protein kinase cascade lasting on the order of minutes. If the signal transduction took say 30 minutes, that's 5 minutes per gap, 400 nm on average per gap.
A test of whether diffusion can suffice:
For an estimated speed of 20 nms-1 of each protein, it would take on the order of 8000 seconds (~100 minutes) to sweep out an arbitrary volume with 400 nm diameter. But at 600 nms-1, it would take only ~300 seconds (~5 minutes). As this would mean the speed of a water molecule is on the order of 15 µms-1, and this is reasonable considering the possible speed of a water molecule, each collision necessary per gap can occur if the concentration (~1 molecule/8 x 108 nm3) of each molecule is high enough. For example, MAP3K1 164 kDa, concentration would be 0.341 ng/ml.
Interaction time decreases with increasing MAP3K1 concentration:
As the number of interactant molecules increases, in this case for MAP3K1, within approximately the same volume, the time to interaction changes inversely. Two molecules of MAP3K1 corresponds to ~150 seconds. Ten to 30 s. That's 3.41 ng/ml of MAP3K1. For a dose of 50 ng/ml (~147 molecules/ml), a minimum interaction time is near 2 s, subject to the dose-response relationship. The table below lists possible speeds, interaction times, and concentrations for 10 molecules of each participant in the transduction to the cytoplasmic side of the nuclear membrane.
| Protein | Mass (Da) | Speed (nm/s) | Time (s) | Concentration (ng/ml) |
|---|---|---|---|---|
| GRB2 | 25k | ~1500 | ~12 | ~0.520 |
| SOS1 | 152k | ~620 | ~29 | ~3.16 |
| RASD1 | 32k | ~1400 | ~13 | ~0.665 |
| RAF1 | 73k | ~900 | ~20 | ~1.52 |
| MAP3K1 | 164k | ~600 | ~30 | ~3.41 |
| MKNK1 | 30k | ~1400 | ~13 | ~0.624 |
| CREB1 | 25k | ~1500 | ~12 | ~0.520 |
The total time for the signal transduction, not including the intranuclear time, is ~2.2 minutes. Additional interactants in the signal cascade when already present in the cytosol, for a similar sized cell would decrease the necessary volume to be travelled, thereby decreasing interaction times per interactant for comparable speeds and concentrations, leaving the total time for the signal transduction similar or possibly shorter.
However, diffusion is a random walk process. One initial stimulus outside the cell will fail. In order for diffusion to succeed, ~106 stimuli distributed all around the cell and the slight focussing effect of the cytoplasmic side of the cell membrane are needed to get one molecule into the nucleus. Increasing the concentration or increasing the number of stimuli increases the number of molecules entering the nucleus.
The focussing effect is simply that at the cell membrane >50% of the possible movements are eliminated, with only those tending inward occurring. Any molecule being moved to leave the cell probably bounces off the membrane inwardly. In addition, diffusion in 3 dimensions is transient; i.e., the RAS molecule will not return to the transmembrane protein.
Physiological transduction[edit | edit source]
In physiology, transduction is the conversion of a stimulus from one form to another.
Transduction in the nervous system typically refers to synaptic events wherein an electrical signal, known as an action potential, is converted into a chemical one via the release of neurotransmitters. Conversely, in sensory transduction a chemical or physical stimulus is transduced by sensory receptors into an electrical signal. Each of these probably involves a loss of energy.
Genetic transduction[edit | edit source]
Genetic transduction is the process by which bacterial DNA is moved from one bacterium to another usually by a virus.
While the form of energy probably does not change, energy is lost and conveyed.
Acknowledgements[edit | edit source]
The content on this page was first contributed by: Henry A. Hoff.
Initial content for this page in some instances came from Wikipedia.
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 Kirschvink JL (1996). "Microwave absorption by magnetite: a possible mechanism for coupling nonthermal levels of radiation to biological systems". Bioelectromagnetics. 17: 187–94.
- ↑ 2.0 2.1 2.2 Schroeder P, Haendeler J, Krutmann J (2008). "The role of near infrared radiation in photoaging of the skin". Exp Gerontol. 43 (7): 629–32. doi:10.1016/j.exger.2008.04.010. PMID 18534799. Unknown parameter
|month=ignored (help) - ↑ Schroeder P, Pohl C, Calles C, Marks C, Wild S, Krutmann J (2007). "Cellular response to infrared radiation involves retrograde mitochondrial signaling". Free Radic Biol Med. 43 (1): 128–35. PMID 17561101. Unknown parameter
|month=ignored (help) - ↑ Schroeder P, Lademann J, Darvin ME, Stege H, Marks C, Bruhnke S, Krutmann J (2008). "Infrared radiation-induced matrix metalloproteinase in human skin: implications for protection". J Invest Dermatol. 128 (10): 2491–7. PMID 18449210. Unknown parameter
|month=ignored (help) - ↑ 5.0 5.1 Schwarz T (1998). "UV light affects cell membrane and cytoplasmic targets". J Photochem Photobiol B. 44 (2): 91–6. PMID 9757589. Unknown parameter
|month=ignored (help) - ↑ Pettersson H, Allison DJ, von Schulthess GK, Smith HJ, ed. (1998). The Encyclopaedia of medical imaging volume I physics, techniques and procedures. Lund, Sweden: ISIS Medical Media The NICER Institute. p. 459. ISBN 82-91942-00-5.
- ↑ Blevis IM, Hunt DC, Rowlands JA (1998). "X-ray imaging using amorphous selenium: Determination of Swank factor by pulse height spectroscopy". Med Phys. 25 (5): 638–41. Unknown parameter
|month=ignored (help) - ↑ Lee YS, Sohn KC, Jang S, Lee Y, Hwang C, Kim KH, Cho MJ, Kim CD, Lee JH (2008). "Anti-apoptotic role of S100A8 in X-ray irradiated keratinocytes". J Dermatol Sci. 51 (1): 11–8. PMID 18325741. Unknown parameter
|month=ignored (help) - ↑ Inan US, Reising SC, Fishman GJ, Horack JM (1996). "On the association of terrestrial gamma-ray bursts with lightning and implications for sprites". Geophysical Res Letters. 23 (9): 1017–20. Unknown parameter
|month=ignored (help) - ↑ Wolf CM, Eastman A (1999). "The Temporal Relationship between Protein Phosphatase, Mitochondrial CytochromecRelease, and Caspase Activation in Apoptosis". Exp Cell Res. 247 (2): 505–13. doi:10.1006/excr.1998.4380. PMID 10066378. Unknown parameter
|month=ignored (help) - ↑ 11.0 11.1 Rothkamm K (2003). "Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses". Proc Natl Acad Sci. USA. 100 (9): 5057–62.
- ↑ Shilnikova DL; et al. (2003). "Cancer mortality risk among workers at the Mayak nuclear complex". Radiation Res. 159 (6): 787–98.
- ↑ Ran XZ; et al. (1998). "Effects of combined radiation and thermal burn injury on the survival of skin allograft and immune function in". Chinese Medical J. 111 (7): 634–7.
- ↑ Randall K; et al. (1992). "The effect of whole-body gamma-irradiation on localized beta-irradiation-induced skin reactions in mice". International J Radiation Biol. 62 (6): 729–33.
- ↑ Friesen C, Lubatschofski A, Kotzerke J, Buchmann I, Reske SN, Debatin KM (2003). "Beta-irradiation used for systemic radioimmunotherapy induces apoptosis and activates apoptosis pathways in leukaemia cells". Euro J Nucl Med Mol Imaging. 30 (9): 1251–61. doi:10.1007/s00259-003-1216-z. PMID 12830326. Unknown parameter
|month=ignored (help) - ↑ 16.0 16.1 16.2 16.3 Esposito D, Faraloni C, Fasolo F, Margonelli A, Torzillo G, Zanini A, Giardi MT (2006). Giardi MT, Piletska EV, eds. Chapter 17: Biodevices for Space Research, in Biotechnological Applications of Photosynthetic Proteins: Biochips, Biosensors and Biodevices. Springer US. pp. 192–208. doi:10.1007/978-0-387-36672-2_17. ISBN 978-0-387-33009-9 (Print) 978-0-387-36672-2 (Online) Check
|isbn=value: invalid character (help).
 KSF
KSF