Trimethoprim microbiology
 From Wikidoc - Reading time: 3 min
From Wikidoc - Reading time: 3 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Microbiology[edit | edit source]
Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the required enzyme, dihydrofolate reductase. This binding is very much stronger for the bacterial enzyme than for the corresponding mammalian enzyme. Thus, trimethoprim selectively interferes with bacterial biosynthesis of nucleic acids and proteins.
Trimethoprim has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
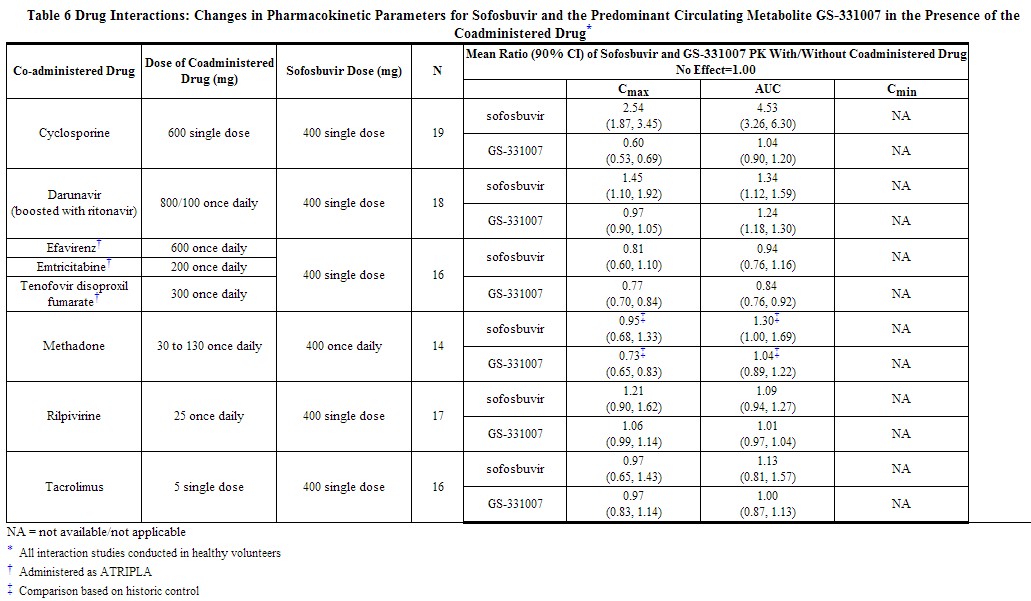 |
Susceptibility Tests[edit | edit source]
Dilution techniques[edit | edit source]
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MIC's). These MIC's provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MIC's should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of trimethoprim powder. The MIC values should be interpreted according to the following criteria:
For testing aerobic microorganisms isolated from urinary tract infections:
 |
When testing Haemophilus influenzae 1
 |
When testing Streptococcus pneumoniae 2
 |
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard trimethoprim3 powder should provide the following MIC values:
 |
Diffusion techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 5 mcg trimethoprim to test the susceptibility of microorganisms to trimethoprim.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 5 mcg trimethoprim4 disk should be interpreted according to the following criteria:
For testing aerobic microorganisms isolated from urinary tract infections:
 |
For testing Haemophilus influenzae 5
 |
Note:
Diffusion techniques are not recommended for determining susceptibility of Streptococcus pneumoniae to trimethoprim.
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for trimethoprim.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 5 mcg trimethoprim4 disk should provide the following zone diameters in this laboratory test quality control strain:
 |
Note:
Diffusion techniques are not recommended for determining susceptibility of Streptococcus pneumoniae to trimethoprim.
4 Blood-containing media (except for lysed horse blood) are generally not suitable for testing trimethoprim. Mueller-Hinton agar should be checked for excessive levels of thymidine. To determine whether Mueller-Hinton medium has sufficiently low levels of thymidine and thymine, an Enterococcus faecalis (ATCC 29212 or ATCC 33186) may be tested with trimethoprim/sulfamethoxazole disks. A zone of inhibition ≥20 mm that is essentially free of fine colonies indicates a sufficiently low level of thymidine and thymine. 5 Interpretative criteria applicable only to tests performed by disk diffusion method using Haemophilus Test Medium (HTM).2[1]
References[edit | edit source]
- ↑ "PRIMSOL (TRIMETHOPRIM HYDROCHLORIDE) SOLUTION [FSC LABORATORIES, INC]". Retrieved 9 January 2014.
Adapted from the FDA Package Insert.
 KSF
KSF