Tuberculosis screening
 From Wikidoc - Reading time: 7 min
From Wikidoc - Reading time: 7 min
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Drug-Susceptible and Drug-Resistant Tuberculosis(ATS 2025 Guidelines) |
|
Case Studies |
|
Tuberculosis screening On the Web |
|
American Roentgen Ray Society Images of Tuberculosis screening |
|
Risk calculators and risk factors for Tuberculosis screening |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mashal Awais, M.D.[2]; Alejandro Lemor, M.D. [3]; Marjan Khan M.B.B.S.[4]
Overview[edit | edit source]
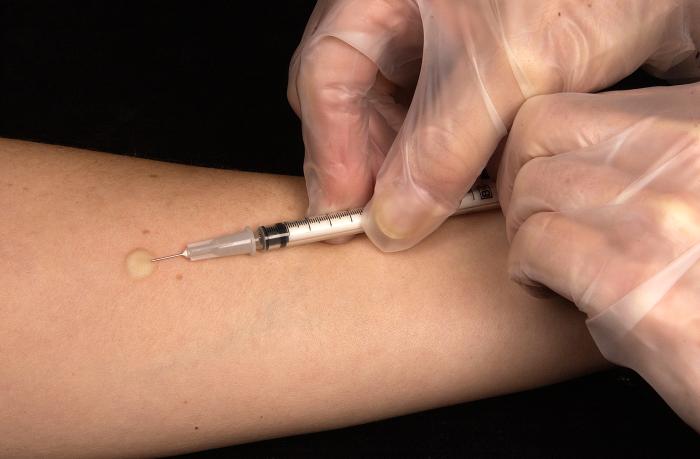
Tuberculosis screening is performed using a mantoux tuberculin skin test, also known as a tuberculin skin test or a PPD. This test is done by intradermal injection of a small amount of a purified protein derivative (PPD) of the tuberculosis bacterium then observing the reaction in the following days.
Screening[edit | edit source]
Mantoux Tuberculin Skin Test[edit | edit source]
- The Mantoux tuberculin skin test (TST) is primarily used to evaluate a person whether they are infected with Mycobacterium tuberculosis.
- The TST is done by intradermal injection 0.1 ml of tuberculin purified protein derivative (PPD) into the inner surface of the forearm. The injection is done using a tuberculin syringe, with the needle bevel facing upward. In case of a correct injection, it produces a pale elevation of the skin (wheal) 6 to 10 mm in diameter.
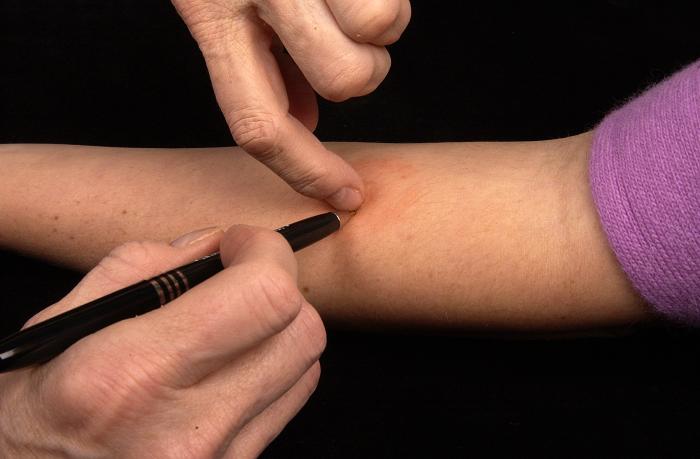
- The reaction is read between 48 and 72 hours after administration. If the patient does not return within 72 hours, another TST should be rescheduled.
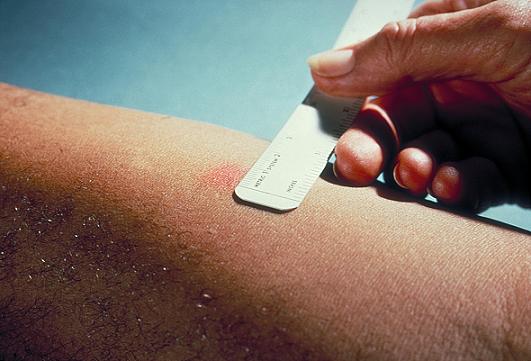
- The induration (palpable, raised, hardened area, or swelling) should be measured in millimeters not the erythema (redness), and the diameter of the indurated area is measured across the forearm (perpendicular to the long axis).
- The only contraindication for TST is in persons who had any severe reaction (e.g., necrosis, blistering, anaphylactic shock, or ulcerations) to another previous TST.
- TST is not contraindicated for any other individuals, including infants, children, pregnant women, HIV-infected patients, or individuals who received BCG vaccination.
- In some individuals with with previous M. tuberculosis infection, the reaction to tuberculin can wane over time, so if TST is done years after infection, it may yield false-negative reaction. However, the TST may activate the immune system, leading to a positive, or boosted reaction to the following tests. Doing a second TST after an initial negative TST reaction, that is known as two-step testing, is preferred especially for healthcare workers or nursing home residents (retested periodically).
Classification of Tuberculin Reaction[edit | edit source]
Interpretation of TST is based on on two elements:
- Measurement of the induration in millimeters.
- The individual’s risk of being infected with TB and also of progression to disease if infected.
   |
| Tuberculin Reaction | Considered a Positive Result in: |
|---|---|
| ≥ 5 mm |
|
| ≥ 10 mm |
|
| ≥ 15 mm |
|
| Table adapted from CDC[1] | |
| False-Positive Reactions | False-Negative Reactions |
|---|---|
In some cases, some individuals react to the TST positively although they are not infected with M. tuberculosis. The causes of these false-positive reactions involve, but are not limited to, the following:
|
On the other hand, Some individuals do not react to the TST although they are infected with M. tuberculosis. The causes for these false-negative reactions involve, but are not limited to, the following:
|
| Table adapted from CDC[1] | |
Recommendations for Human Immunodeficiency Virus (HIV) Screening in Tuberculosis Clinics Adapted from CDC[2][edit | edit source]
- According to CDC, HIV screening is recommended for all TB patients after the patient notification that testing will be done unless the patient defers (i.e., opt-out screening). This includes patients with TB disease and with latent TB infection.
- HIV testing is also recommended for individuals who are suspected of having TB disease, patients with latent TB infection, and contacts of TB patients.
- Prevention counseling and separate written consent for HIV testing are no longer necessary.
- These recommendations are effective for eliminating missed opportunities for HIV screening and reducing significant barriers to HIV testing in health care settings by:
- Opt-out screening refers to performing HIV testing after informing the patient that the test will be done, and despite being declined by the patient, it is strongly recommended. Assent is inferred unless the patient defers HIV testing.
Quantiferon Gold testing[edit | edit source]
- The early identification of Mycobacterium tuberculosis infection is a complex issue regarding the control and prevention of TB.[3]
- Over several years, the tuberculin skin test (TST) was the most commonly used test for the diagnosis of TB infection due to its low cost and convenience in most countries.[4]
- However, PPD testing has several disadvantages, including low specificity in individuals who received the Bacillus Calmette-Guerin (BCG) vaccination.[5]
- In the last decade, interferon gamma release assays (IGRAs) have been used to detect Latent Tuberculosis.[6]
- Interferon-gamma release assays (IGRAs) are immunodiagnostics techniques that measure interferon-gamma (IFN-γ) released by T-cells in response to Mycobacterium tuberculosis-specific antigen.[7]
- There are two commercial in vitro IGRAs; including the QuantiFERON-TB Gold In-Tube (QFT-GIT) test and T-SPOT.TB that have been used for for the early detection of M. tuberculosis infection.[7]
- QuantiFERON-TB Gold includes proteins that are almost exclusively found in mycobacterium tuberculosis.[8]
- The QuantiFERON-TB Gold In-Tube test (QFT-GIT) assay measures the IFN-γ serum concentration following stimulation by specific TB antigens.[8]
- The Quantiferon Gold test is used on a large scale in developed countries.[6]
- The ability of QFT-GIT will be to monitor the response to anti-tuberculosis treatment is not fully-understood.[9]
| Category | 2005 Recommendation | 2019 Recommendation |
|---|---|---|
| Baseline (preplacement) screening and testing | TB screening of all HCP, in addition to a symptom evaluation and testing (TST or IGRA) for those without documented previous TB disease or LTBI. | TB screening of all HCP, in addition to a symptom evaluation and testing (TST or IGRA) for those without documented previous TB disease or LTBI (unchanged); individual TB risk assessment (new). |
| Postexposure screening and testing | Symptom evaluation for all HCP if exposure is identified. For HCP having a baseline negative TB test and no previous TB disease or LTBI, do a test (IGRA or TST) when the exposure is detected. If that test is negative, perform another test 8–10 weeks following the last exposure. | Symptom evaluation for all HCP if exposure is identified. For HCP having a baseline negative TB test and no previous TB disease or LTBI, do a test (IGRA or TST) when the exposure is detected. If that test is negative, perform another test 8–10 weeks following the last exposure (unchanged). |
| Serial screening and testing for HCP without LTBI | According to health care facility and setting risk assessment. Not recommended for HCP working in low-risk health care settings. Recommended for HCP working in medium-risk health care settings and settings with potential ongoing transmission. | Not routinely recommended (new); can consider for selected HCP groups (unchanged); recommend annual TB education for all HCP (unchanged), including information about TB exposure risks for all HCP (new emphasis). |
| Evaluation and treatment of positive test results | Referral to determine whether LTBI treatment is indicated. | Treatment is encouraged for all HCP with untreated LTBI, unless medically contraindicated (new). |
| Abbreviations: IGRA = interferon-gamma release assay; LTBI = latent tuberculosis infection; TST = tuberculin skin test.
| ||
References[edit | edit source]
- ↑ 1.0 1.1 "CDC Tuberculin Skin Testing".
- ↑ "CDC Recommendations for Human Immunodeficiency Virus (HIV) Screening in Tuberculosis (TB) Clinics".
- ↑ "WHO | Global tuberculosis report 2018, SYSTEM DO NOT MOVE OR EDIT".
- ↑ Houben RM, Dodd PJ (October 2016). "The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling". PLoS Med. 13 (10): e1002152. doi:10.1371/journal.pmed.1002152. PMC 5079585. PMID 27780211.
- ↑ Detjen AK, Keil T, Roll S, Hauer B, Mauch H, Wahn U, Magdorf K (August 2007). "Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis". Clin. Infect. Dis. 45 (3): 322–8. doi:10.1086/519266. PMID 17599309.
- ↑ 6.0 6.1 Lu P, Chen X, Zhu LM, Yang HT (June 2016). "Interferon-Gamma Release Assays for the Diagnosis of Tuberculosis: A Systematic Review and Meta-analysis". Lung. 194 (3): 447–58. doi:10.1007/s00408-016-9872-5. PMID 27039307.
- ↑ 7.0 7.1 Lalvani A (June 2007). "Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy". Chest. 131 (6): 1898–906. doi:10.1378/chest.06-2471. PMID 17565023.
- ↑ 8.0 8.1 Dilektasli AG, Erdem E, Durukan E, Eyüboğlu FÖ (November 2010). "Is the T-cell-based interferon-gamma releasing assay feasible for diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country?". Jpn. J. Infect. Dis. 63 (6): 433–6. PMID 21099095.
- ↑ Dheda K, Pooran A, Pai M, Miller RF, Lesley K, Booth HL, Scott GM, Akbar AN, Zumla A, Rook GA (August 2007). "Interpretation of Mycobacterium tuberculosis antigen-specific IFN-gamma release assays (T-SPOT.TB) and factors that may modulate test results". J. Infect. 55 (2): 169–73. doi:10.1016/j.jinf.2007.02.005. PMID 17448540.
 KSF
KSF