(Trimethylsilyl)methyllithium
 From Wikipedia - Reading time: 3 min
From Wikipedia - Reading time: 3 min
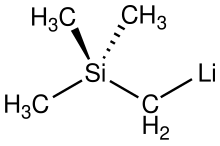
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.157.622 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H11LiSi | |
| Molar mass | 94.16 g·mol−1 |
| Appearance | white or colorless solid |
| Density | 0.937 g/cm3 |
| Hazards | |
| GHS labelling:[1] | |
 
| |
| Danger | |
| H225, H314 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2tms. It crystallizes as the hexagonal prismatic hexamer [LiCH2tms]6, akin to some polymorphs of methyllithium.[2] Many adducts have been characterized including the diethyl ether complexed cubane [Li4(μ3-CH2tms)4(Et2O)2][3] and [Li2(μ-CH2tms)2(tmeda)2].[4]
Preparation[edit]
(Trimethylsilyl)methyllithium, which is commercially available as a THF solution, is usually prepared by treatment of [(trimethylsilyl)methyl chloride with butyllithium:[5]
- (CH3)3SiCH2Cl + BuLi → (CH3)3SiCH2Li + BuCl
Trimethylsilylmethyl magnesium chloride is often functionally equivalent to trimethylsilylmethyllithium. It is prepared by the Grignard reaction of trimethylsilylmethyl chloride.[6][7]
Use in methylenations[edit]
In one example of the Peterson olefination, (trimethylsilyl)methyllithium reacts with aldehydes and ketones to give the terminal alkene (R1 = Me, R2 & R3 = H):
Metal derivatives[edit]
Trimethylsilylmethyllithium is widely used in organotransition metal chemistry to affix trimethylsilylmethyl ligands. Such complexes are usually produced by salt metathesis involving metal chlorides. These compounds are often highly soluble in nonpolar organic solvents. These complexes enjoy stability because trimethylsilylmethyl ligands are bulky and they resist beta-hydride elimination. In these regards, trimethylsilylmethyl is akin to neopentyl.
Related compounds[edit]
References[edit]
- ^ "(Trimethylsilyl)methyllithium". pubchem.ncbi.nlm.nih.gov. Retrieved 12 January 2022.
- ^ Tecle', Berhan; Maqsudur Rahman, A.F.M.; Oliver, John P. (1986). "X-ray Crystal Structure of Trimethylsilylmethyllithium". Journal of Organometallic Chemistry. 317 (3): 267–275. doi:10.1016/0022-328X(86)80537-X.
- ^ Tatic, Tanja; Meindl, Kathrin; Henn, Julian; Pandey, Sushil Kumar; Stalke, Dietmar (2010). "The First Asymmetric Organolithium Tetramers with Simple Ether Donor Bases". Chemical Communications. 46 (25): 4562–4564. doi:10.1039/c002504f. PMID 20502820.
- ^ Tatic, Tanja; Ott, Holger; Stalke, Dietmar (2008). "Deaggregation of Trimethylsilylmethyllithium". European Journal of Inorganic Chemistry. 2008 (24): 3765–3768. doi:10.1002/ejic.200800610.
- ^ Age, David J.; Werth, Jacob (2019). "Trimethylsilylmethyllithium". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt321. ISBN 978-0471936237.
- ^ Brennan, David J.; Graaskamp, James M.; Dunn, Beverly S.; Allcock, Harry R. (2007) [1989]. Organosilicon Derivatives of Cyclic and High Polymeric Phosphazenes. Inorganic Syntheses. pp. 60–68. doi:10.1002/9780470132562.ch15. ISBN 9780470132562.
- ^ Shioiri, Takayuki; Aoyama, Toyohik; Mori, Shigehiro (1990). "Trimethylsilyldiazomethane". Organic Syntheses. 68: 1. doi:10.15227/orgsyn.068.0001.
- ^ Li, Xiaofang; Nishiura, Masayoshi; Hu, Lihong; Mori, Kyouichi; Hou, Zhaomin (2009). "Alternating and Random Copolymerization of Isoprene and Ethylene Catalyzed by Cationic Half-Sandwich Scandium Alkyls". Journal of the American Chemical Society. 131 (38): 13870–13882. doi:10.1021/ja9056213. PMID 19728718.
- ^ Jerry L. Atwood; Torgny Fjeldberg; Michael F. Lappert; N. Tuyet Luong-Thi; Riz Shakir; Andrew J. Thorne (1984). "Molecular structures of Bis(trimethylsilyl)methyl-lithium [(LiR), R = CH(SiMe3)2] in the Vapour (Gas-Phase Electron Diffraction: a Monomer, n= 1) and the Crystal (X-ray: a Polymer, n=∞)". Journal of the Chemical Society, Chemical Communications (17): 1163–1165. doi:10.1039/C39840001163.
- ^ Colin Eaborn; Peter B. Hitchcock; J. David Smith; Alice C. Sullivan (1983). "Crystal structure of the Tetrahydrofuran Adduct of Tris(trimethylsilyl)-Methyl-Lithium, [Li(thf)4][Li{C(SiMe3)3}2], an Ate Derivative of Lithium". Journal of the Chemical Society, Chemical Communications: 827–828. doi:10.1039/C39830000827.
 KSF
KSF